



Polymers, a broad category of materials, are made up of many small molecules called monomers that are bonded together to form long chains and are utilized in a wide range of products and goods. People have been using polymers in their lives for a long time, but they did not fully understand them until World War II. For the fabrication of the article required for civilized life, there were relatively few resources accessible.
The term “Polymer” has its origin in the Greek word “polus meros” which means many (polus) parts (meros). Accordingly, Polymers are materials produced by a repeated chain of molecules.
Plastics, which are manufactured polymers, are frequently referred to as polymers. On the other hand, natural polymers exist; rubber and wood, for example, are natural polymers made up of isoprene, a simple hydrocarbon, according to Encyclopedia Britannica. Proteins are polymers of amino acids, while nucleic acids (DNA and RNA) are polymers of nucleotides, which are complicated compounds made up of nitrogen-containing bases, sugars, and phosphoric acid, among other things.
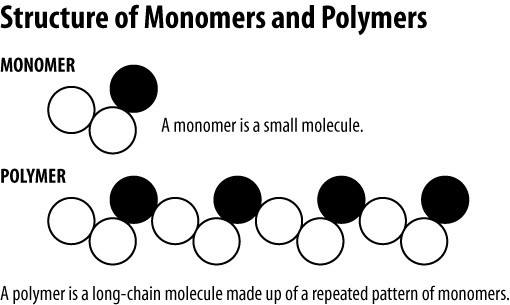
Polymers can be classified in various ways due to their large quantity and diverse behaviors and the fact that they can be found naturally or synthesized.
Natural polymers, synthetic polymers, and semi-synthetic polymers are the three types of polymers included in this category.
Natural Polymers
Polymers can be found in nature or created in a laboratory. Long before they understood in the chemistry lab, natural polymers were employed for their chemical properties: Clothing was made from wool, leather, and flax fibers, and animal bone cooked down to form glues. Natural polymers include- i) proteins such as hair, nails, and tortoiseshell ii)Cellulose is found in both paper and trees iii) DNA iv) pitch v)Starches in plants vi) wool vii) silk and viii) natural rubbers.
Synthetic Polymers
Human-made polymers are known as synthetic polymers. They are divided into four categories based on their utility:
Thermoplastics are polymers that become moldable and malleable after reaching a particular temperature then harden when cooled. On the other hand, Thermosets harden and can’t change shape once they’ve set, which is why they’re commonly employed in adhesives.
An elastomer is a flexible polymer that is used interchangeably with rubber. Synthetic fibers are a large polymer category that improves natural plant and animal fibers. Fully synthetic polymers include-
Semi-Synthetic Polymers
They are made from naturally existing polymers that have been chemically modified. Examples of semi-synthetic polymers are cellulose nitrate, cellulose acetate, etc.


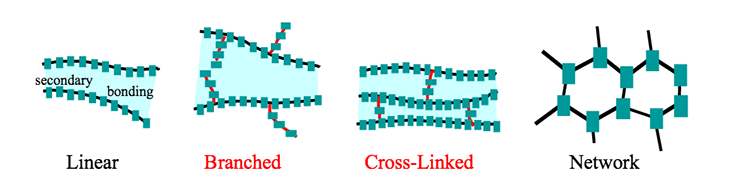 Depending on the structure, there are three types of polymers as mentioned below:
Depending on the structure, there are three types of polymers as mentioned below:
Linear structure polymers
The structure of these polymers resembles a long straight chain with similar links linking them. These are made up of monomers bonded together to form a lengthy chain. The melting temperature and density of these polymers are higher than those of other polymers. PVC is a good example of this (Poly-vinyl chloride). This polymer is commonly utilized in the manufacture of electrical cables and pipes.
Cross-linked Polymers
Monomers are joined together to form a three-dimensional network in this type of polymer. Because they are made up of bi-functional and tri-functional molecules, the monomers have strong covalent bonds. These polymers are hard to work with because they are brittle.
Branch Chain Polymers
The structure of these polymers is similar to branches arising at random locations from a single linear chain, as the label suggests. Monomers unite to form a long straight chain with various-length branching chains. Because of their branches, the polymers are not closely packed together. They have a low melting point and a low density. Low-density polyethylene (LDPE) is a common example used in plastic bags and general-purpose containers.
Polymerization is a chemical reaction in which monomer molecules are combined to form a polymer chain (or three-dimensional networks). Polymers are classed as follows based on the type of polymerization:
Condensation Polymers:
These polymers combine monomers and remove tiny molecules such as water and alcohol. In these types of condensation processes, the monomers are bi-functional or tri-functional. The polymerization of hexamethylenediamine and adipic acid to produce Nylon – 66 is a common example in which water molecules are removed throughout the process.
Strong covalent bonds connect atoms in individual polymer molecules in polymers. Polymer molecules are drawn together by intermolecular interactions (forces between molecules). It’s worth noting that the intensity of the interactions between molecules determines the properties of solid materials like polymers. Polymers can be classified into four types using this method.
Rubber-like solid polymers with a high degree of elasticity are known as elastomers. When we say elastic, we’re referring to the fact that a modest amount of force may easily stretch the polymer. Rubber bands are the most common examples (or hair bands). Applying a little pressure on the band lengthens it.
The weakest intermolecular forces hold the polymer chains together, allowing the polymer to be stretched. However, as you can see, reducing the stress causes the rubber band to revert to its natural shape. This occurs when we insert crosslinks between the polymer chains, which aid in the polymer’s retraction and return to its original shape. Vulcanized rubber is used to make our car tires. This is when sulfur is introduced to crosslink the polymer chains.
Thermosetting plastics are semi-fluid polymers with low molecular weights. When heated, they cause cross-linking between polymer chains, making them stiff and infusible. They form a three-dimensional structure when heat is applied. This reaction is irreversible in nature. The most popular thermosetting polymer is bakelite, which is used to produce electrical insulation.
iii) Thermoplastics:
Thermoplastic polymers are long-chain polymers held together by intermolecular interactions. When heated, these polymers soften (like a thick fluid) and stiffen when allowed to cool, forming a rigid mass. They don’t have any cross bonds and may be molded using heat and molds. Polystyrene, also known as PVC, is a popular example (which is used in making pipes).
According to polymer classification, these are polymer types that resemble a natural thread and can be easily woven. They have low elasticity and high tensile strength due to strong inter-molecule tensions between the chains. Hydrogen bonds or dipole-dipole interactions could be the intermolecular forces. The melting point of fibers is both high and acute. Nylon-66, which is extensively used in carpets and clothing, is an excellent example.
Applications of polymers are multi-dimensional. Some of the common uses of polymers are listed below:
© 2024 iasgyan. All right reserved