Description
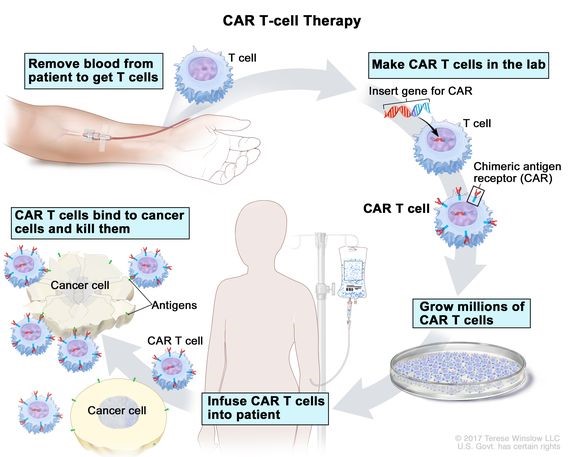
Source: Cancer.gov
Disclaimer: Copyright infringement not intended.
Context
- The President of India launched India’s first home-grown gene therapy for cancer at IIT Bombay.
- India’s first CAR-T cell therapy is developed through collaboration between the Indian Institute of Technology, Bombay and Tata Memorial Hospital in association with industry partner ImmunoACT.
Details
About CAR-T Cell Therapy
- CAR-T cell therapy, or chimeric antigen receptor T-cell therapy, is a type of immunotherapy that harnesses the power of the immune system to target and destroy cancer cells.
- It involves genetically modifying a patient's own T cells to express chimeric antigen receptors (CARs), which enable the T cells to recognize and attack cancer cells with specific surface antigens.
Mechanism of Action:
- T cells are collected from the patient's blood through leukapheresis and genetically engineered to express CARs targeting a specific antigen present on cancer cells.
- CARs consist of an extracellular antigen recognition domain, a transmembrane domain, and an intracellular signaling domain.
- Upon infusion back into the patient, CAR-T cells recognize cancer cells expressing the target antigen and initiate a cytotoxic immune response, leading to the destruction of cancer cells.
|
Introduction to T Cells
- T cells are a type of white blood cell (lymphocyte) that plays a crucial role in the immune system.
- They are produced in the bone marrow and mature in the thymus gland, hence the name "T" cells.
- T cells are central players in adaptive immunity, which involves a targeted response to specific pathogens.
Types of T Cells:
- Helper T Cells (CD4+ T Cells):
- Assist other immune cells in mounting an effective immune response.
- Coordinate the activities of various immune cells by secreting signaling molecules called cytokines.
- Differentiate into subsets such as Th1, Th2, Th17, and Treg cells, each with specific functions.
- Cytotoxic T Cells (CD8+ T Cells):
- Recognize and destroy infected or abnormal cells, including virus-infected cells and cancer cells.
- Release cytotoxic molecules such as perforin and granzymes to induce apoptosis (cell death) in target cells.
- Memory T Cells:
- Formed after an initial immune response to a pathogen.
- Provide long-lasting immunity by "remembering" previous encounters with specific antigens and mounting a rapid response upon re-exposure.
- Regulatory T Cells (Tregs):
- Suppress excessive immune responses to prevent autoimmune reactions and maintain immune tolerance.
- Play a critical role in preventing immune-mediated tissue damage.
|
Development and Manufacturing Process:
- T cells are isolated from the patient's blood sample and activated using cytokines such as interleukin-2 (IL-2).
- The T cells are then genetically modified using viral vectors (such as lentiviral or retroviral vectors) to introduce the CAR gene into their genome.
- The modified CAR-T cells are expanded in vitro to achieve sufficient numbers for therapeutic infusion.
- Quality control tests are performed to ensure the safety, purity, and potency of the CAR-T cell product before administration to the patient.
Clinical Applications:
- CAR-T cell therapy has shown remarkable efficacy in treating certain types of hematologic malignancies, including:
- Acute lymphoblastic leukemia (ALL)
- B-cell non-Hodgkin lymphoma (NHL), such as diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma
- Chronic lymphocytic leukemia (CLL)
- Ongoing research is exploring the potential of CAR-T cell therapy in treating solid tumors, such as glioblastoma, ovarian cancer, and pancreatic cancer.
Side Effects:
- CAR-T cell therapy can be associated with potentially severe side effects, known as cytokine release syndrome (CRS) and neurologic toxicities.
- CRS is characterized by fever, flu-like symptoms, hypotension, and organ dysfunction due to the release of pro-inflammatory cytokines by activated T cells.
- Neurologic toxicities may include confusion, delirium, seizures, and aphasia, resulting from immune-mediated inflammation in the central nervous system.
- Prompt recognition and management of side effects are essential, typically involving supportive care, anti-cytokine therapies (such as tocilizumab and corticosteroids), and close monitoring in an intensive care setting if necessary.

About Cancer
- Cancer is a complex group of diseases characterized by abnormal cell growth and proliferation.
- It can arise in any tissue or organ of the body when normal cellular processes regulating growth, division, and death are disrupted, leading to uncontrolled cell growth and tumor formation.
Types of Cancer:
- Carcinomas: Arise from epithelial cells lining the surfaces or cavities of organs, such as lung, breast, prostate, and colon cancers.
- Sarcomas: Originate from connective tissues, including bone, muscle, and cartilage.
- Leukemias: Develop in blood-forming tissues, such as bone marrow, and result in abnormal proliferation of white blood cells.
- Lymphomas: Affect the lymphatic system, leading to the abnormal growth of lymphocytes (white blood cells).
- Central Nervous System (CNS) Tumors: Occur in the brain or spinal cord, including gliomas, meningiomas, and medulloblastomas.
Causes and Risk Factors:
- Genetic Factors: Inherited mutations in certain genes, such as BRCA1 and BRCA2, increase the risk of developing certain types of cancer.
- Environmental Factors: Exposure to carcinogens, such as tobacco smoke, ultraviolet radiation, asbestos, and certain chemicals, can increase cancer risk.
- Lifestyle Factors: Unhealthy lifestyle habits, including smoking, excessive alcohol consumption, poor diet, lack of physical activity, and obesity, contribute to cancer development.
- Viral Infections: Infection with oncogenic viruses, such as human papillomavirus (HPV), hepatitis B virus (HBV), and Epstein-Barr virus (EBV), can lead to the development of specific cancers.
About Gene Therapy
- Gene therapy is a revolutionary approach aimed at treating genetic disorders by modifying or replacing defective genes.
- It holds the potential to address a wide range of diseases, including inherited disorders, cancers, and certain viral infections, by correcting the underlying genetic abnormalities.
Types of Gene Therapy:
- Gene Addition Therapy: Involves introducing a functional copy of a gene into cells to compensate for a defective or missing gene.
- Gene Editing Therapy: Utilizes molecular tools such as CRISPR-Cas9 to precisely modify the DNA sequence of target genes, correcting mutations or disrupting disease-causing genes.
- Gene Silencing Therapy: Involves suppressing the expression of specific genes using RNA interference (RNAi) or antisense oligonucleotides to treat conditions caused by overactive genes.
Steps in Gene Therapy:
- Identification of Target Gene: The defective gene responsible for the disease is identified through genetic testing and molecular analysis.
- Gene Delivery: A delivery system, often using viral vectors or non-viral methods like nanoparticles, is employed to transport therapeutic genes into target cells.
- Gene Expression: Once inside the cells, the therapeutic gene integrates into the genome or produces functional proteins, restoring normal cellular function.
- Monitoring and Follow-Up: Patients are monitored for therapeutic efficacy and potential side effects, with long-term follow-up to assess the durability of treatment effects.
Applications of Gene Therapy:
- Monogenic Disorders: Gene therapy shows promise in treating single-gene disorders such as cystic fibrosis, sickle cell disease, muscular dystrophy, and hemophilia.
- Cancer Treatment: Oncolytic viruses and gene-editing techniques are being investigated as potential therapies for various cancers, targeting specific genetic mutations or enhancing immune responses against tumor cells.
- Neurological Disorders: Gene therapy holds potential for treating neurological conditions like Parkinson's disease, Alzheimer's disease, and spinal muscular atrophy by delivering therapeutic genes to affected brain cells.
- Infectious Diseases: Gene therapy approaches are being explored to combat viral infections such as HIV/AIDS, hepatitis, and COVID-19 by disrupting viral replication or enhancing immune responses.
Challenges:
- Delivery Efficiency: Ensuring effective delivery of therapeutic genes to target cells while minimizing off-target effects and immune responses remains a major challenge.
- Immune Response: The body's immune system may recognize viral vectors or foreign DNA, leading to immune reactions that limit the effectiveness of gene therapy.
- Long-Term Safety: Potential risks, including insertional mutagenesis, off-target effects, and unintended gene silencing, necessitate rigorous safety assessments and long-term monitoring of patients.
- Ethical and Social Implications: Ethical considerations surrounding gene editing, germline modifications, and equitable access to gene therapy raise complex societal issues that require careful consideration.

Conclusion
- Gene therapy represents a promising paradigm shift in medicine, offering targeted and potentially curative treatments for a wide range of genetic and acquired diseases.
- While significant progress has been made in the development of gene therapy approaches, ongoing research and innovation are essential to address remaining challenges and realize the full potential of gene therapy in improving human health.
Sources:
PIB
Cancer.gov
|
PRACTICE QUESTION
Q. Continued research and clinical development are essential to unlock the full potential of CAR-T cell therapy. Discuss. (250 Words)
|