Description
Disclaimer: Copyright infringement not intended.
In News
- India has approved two more COVID-19 vaccines and the antiviral drug Molnupiravir under emergency use authorisation.
- Currently, India uses Covishield, Covaxin and Sputnik V for vaccination.
About Corbevax
- Corbevax has the distinction of being the first indigenously developed RBD protein sub-unit vaccine in India.
- The vaccine has been approved by the World Health Organization (WHO) under its Emergency Use Listing and, therefore, will be available globally as part of the COVAX initiative to ensure that at least 40% of world is vaccinated on priority.
- The vaccine should be administered intramuscularly in two doses of 0.5 ml each with an interval of 28 days (Day 0 and 28) and has to be stored between 2 degrees Celsius to 8 degrees Celsius.
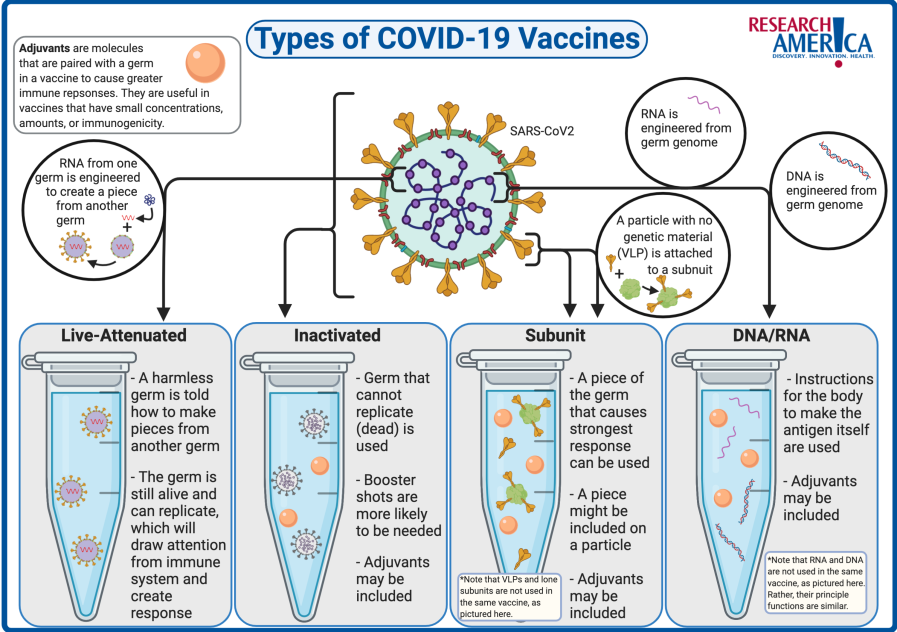
- Corbevax, as mentioned earlier is a protein subunit vaccine, and Covovax, is a nanoparticle-based vaccine.
Trivia
Nanoparticles for vaccine delivery
- As compared to conventional vaccine approaches, nano carrier-based delivery systems offer several advantages including enhanced protection against premature degradation, good stability, and improved adjuvant qualities.
- When used to encapsulate or coat the surface of an antigen, nanocarriers can protect the immunogen from premature proteolytic degradation.
- In addition to their protective qualities, nanocarriers can also improve the specificity of antigen delivery and increase the duration of antigen presentation to these cells needed to achieve long-term immunity.
Other drugs
- Molnupiravir, which was approved this month by the U.S. Food and Drugs Administration (USFDA), along with Paxlovid by Pfizer Inc, is said to be a promising drug for those with mild and moderate disease and also easily administered as a pill. Thirteen companies in India are set to manufacture it.
https://epaper.thehindu.com/Home/ShareArticle?OrgId=GT49BCL0C.1&imageview=0
















