




Disclaimer: Copyright infringement not intended.
Context
Details
Final word
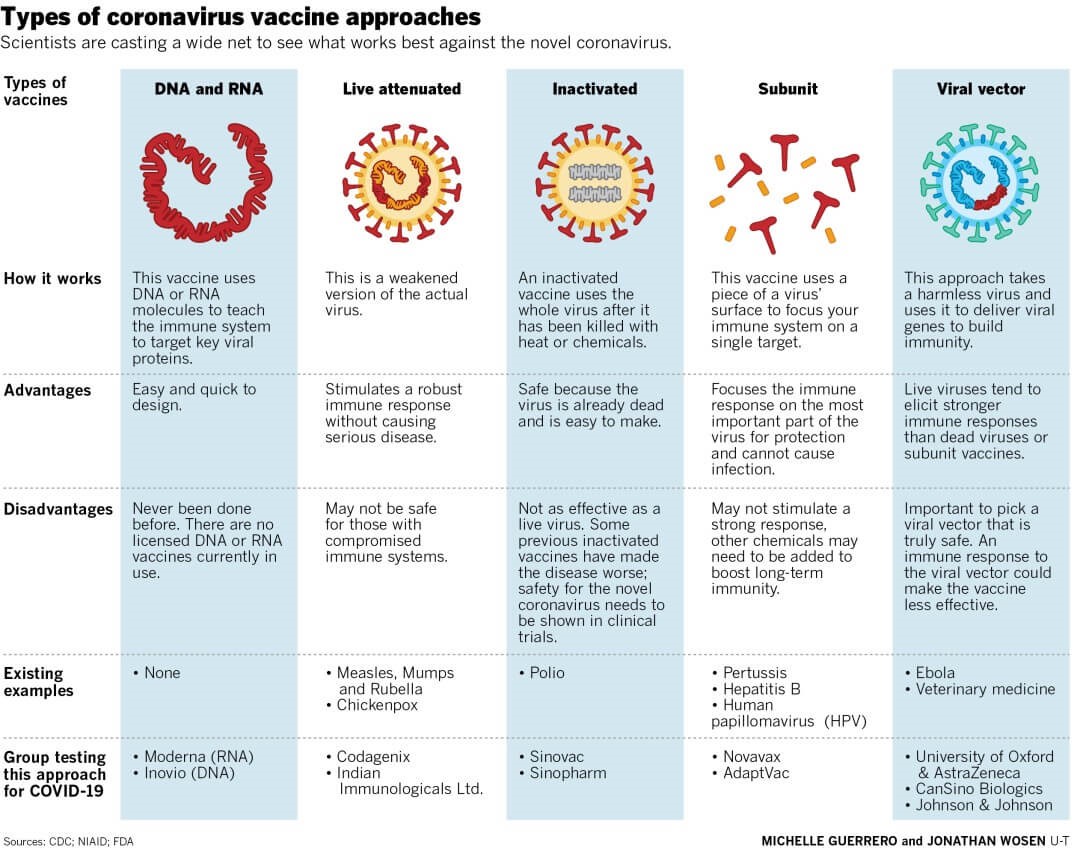
Read: https://www.iasgyan.in/blogs/types-of-vaccines
https://www.iasgyan.in/daily-current-affairs/booster-dose





© 2025 iasgyan. All right reserved