Description
Disclaimer: Copyright infringement not intended.
Context
- ISRO has successfully tested a 100 W class Polymer Electrolyte Membrane Fuel Cell based Power System (FCPS) in its orbital platform, POEM3, launched onboard PSLV-C58 on January 1, 2024.
Details
- The objective of the experiment was to assess Polymer Electrolyte Membrane Fuel cell operation in space and to collect data to facilitate the design of systems for future missions.
- During the short duration test onboard POEM, 180 W power was generated from Hydrogen and Oxygen gases stored on onboard in high pressure vessels.
- It provided a wealth of data on performance of various static and dynamic systems that formed part of the power system and the physics at play.
About Fuel Cells
- Fuel cells are electrochemical devices that convert the chemical energy stored in a fuel directly into electrical energy through a controlled chemical reaction.
- They operate similar to batteries but do not require recharging. Instead, they continue to produce electricity as long as fuel is supplied, offering a promising alternative to internal combustion engines in various applications.

How Fuel Cells Work:
Basic Components:
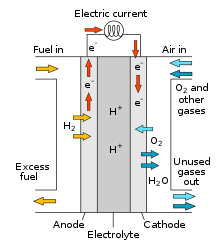
- Anode: Where fuel (commonly hydrogen) is oxidized into protons and electrons.
- Cathode: Where oxygen from the air reacts with protons and electrons.
- Electrolyte: Allows ions (e.g., H+ ions) to pass through but blocks electrons.
- Electrochemical Reaction: Hydrogen (H2) at the anode splits into protons (H+) and electrons (e-). Protons travel through the electrolyte to the cathode, while electrons follow an external circuit, generating electricity. At the cathode, oxygen (O2) combines with protons and electrons to form water (H2O).
Fuel Sources:
- Hydrogen Fuel Cells: Utilize pure hydrogen as the primary fuel, producing only water and heat as byproducts.
- Reforming Fuels: Hydrocarbons like natural gas or methanol can be reformed into hydrogen to power fuel cells, producing less emissions compared to combustion engines.
- Direct Methanol Fuel Cells (DMFCs): Use methanol as a liquid fuel source, offering higher energy density but with certain technical challenges.
Types of Fuel Cells:
- Proton Exchange Membrane Fuel Cells (PEMFCs):
- Commonly used in vehicles and portable applications due to their compactness and quick startup.
- Employ a solid polymer electrolyte membrane that conducts protons while blocking electrons.
- Solid Oxide Fuel Cells (SOFCs):
- Operate at high temperatures (800-1000°C), enabling them to use various fuels, including hydrocarbons and biofuels.
- Suitable for stationary power generation in industries and residential settings due to their high efficiency.
- Molten Carbonate Fuel Cells (MCFCs):
- Operate at higher temperatures (600-700°C), allowing them to internally reform certain fuels.
- Used for large-scale power generation in industries and some utility plants.
- Alkaline Fuel Cells (AFCs):
- Historically used in space missions due to their high efficiency and ability to handle non-pure hydrogen sources.
- Operating on potassium hydroxide (KOH) electrolyte, they are being revisited for niche applications.
Advantages of Fuel Cells:
- Efficiency: Fuel cells offer high energy efficiency, especially in combined heat and power (CHP) applications.
- Environmental Impact: They produce minimal pollutants, with water vapor being the primary emission.
- Versatility: Can operate using various fuel sources, including hydrogen, natural gas, methanol, and biofuels.
- Quiet Operation: Unlike internal combustion engines, fuel cells operate silently.
- Scalability: Suitable for diverse applications, from small portable devices to large-scale power generation.
Challenges:
- Cost: Currently, fuel cell systems are relatively expensive to produce and require further cost reduction for widespread adoption.
- Infrastructure: Developing a robust hydrogen infrastructure for storage, transportation, and distribution is crucial.
- Durability: Improving the longevity and robustness of fuel cell components is essential to increase their lifespan and reliability.
.jpg)
Conclusion
Fuel cells hold tremendous potential as clean and efficient energy conversion devices, with ongoing research focusing on improving their performance, reducing costs, and expanding their applicability across industries and transportation sectors. Continued advancements in materials, manufacturing techniques, and infrastructure will play a pivotal role in their widespread adoption and commercialization.
|
PRACTICE QUESTION
Q. How do fuel cells offer a viable solution in addressing energy needs while mitigating environmental impacts? Discuss with relevant examples. (250 Words)
|
















