Description
Disclaimer: Copyright infringement not intended.
Context
- Recently, the Cabinet approved signing of MoU between India and Suriname in the field of the regulation of medicines.
Highlights of the MoU
- The parties recognize the importance of developing close cooperation and exchanging information in the field of the regulation of medicines in accordance with their respective laws and regulations and reach the following understandings:
- to accept Indian Pharmacopoeia (IP) as a book of standards for medicines in Suriname so as to ensure quality of medicines being manufactured and/or imported in Suriname;
- to accept the Certificate of Analysis issued by Indian manufacturers per IP and to do away with requirement of duplicate testing of the medicines in Suriname;
- to get IPRS and Impurity standards from IPC at reasonably low cost to be used during the quality control analysis;
- to have better scope for development of generic medicines and contributing to availability of affordable medicines in Suriname;
- to promote an understanding of pharmacopoeia in the regulatory framework, requirements and processes;
- to facilitate the exchange of information and documentation relating to the development of monographs of IP;
- to enhance the ability of regulatory authorities in the provision of their services relating to or in connection with public health, to meet the needs of their respective population;
- to explore opportunities for technical cooperation in areas of mutual benefit in the development of monographs and future technologies.
Significance
- The Memorandum of Understanding will facilitate the export of medical products leading to foreign exchange earnings.
- This would be a step towards an Atmanirbhar Bharat.

Benefits of International recognition of the Indian pharmacopoeia! Standards
- It would boost the export of Indian pharmaceutical products to these countries as it would remove double regulation, duplication in testing and post-importation checks. Indian drug exporters would thus, gain a competitive edge and trade would become more remunerative.
- Further, importing nations would gain access to quality Indian medical products at affordable prices.
- Manufacturers in importing countries would have better scope for the development of generic medicines contributing to the availability of affordable medicines to their citizens.
- The various reference standards and impurity standards would become available to these manufacturers at a reasonable cost.
- Convergence in the regulatory practices could help in increasing export of medicines from India and consequentially help in better employment opportunities for educated professionals in the Pharmaceutical sector.
[Note: The Indian Pharmacopoeia (IP) is officially recognized by five (5) countries: Afghanistan, Ghana, Nepal, Mauritius and the Republic of Suriname. The Ministry seeks to expand the nations which recognize the IP.]
|
INDIAN PHARMACOPOEIA COMMISSION (IPC)
Indian Pharmacopoeia Commission (IPC) is an Autonomous Institution of the Ministry of Health and Family Welfare, Govt. of India. IPC is created to set standards of drugs in the country. Its basic function is to update regularly the standards of drugs commonly required for treatment of diseases prevailing in this region. It publishes official documents for improving Quality of Medicines by way of adding new and updating existing monographs in the form of Indian Pharmacopoeia (IP). It further promotes rational use of generic medicines by publishing National Formulary of India. IP prescribes standards for identity, purity and strength of drugs essentially required from health care perspective of human beings and animals. IPC also provides IP Reference Substances (IPRS) which act as a finger print for identification of an article under test and its purity as prescribed in IP.
INDIAN PHARMACOPOEIA (IP)
Indian Pharmacopoeia (IP) is published by the Indian Pharmacopoeia Commission (IPC) on behalf of the Ministry of Health & Family Welfare, Government of India in fulfillment of the requirements of the Drugs and Cosmetics Act, 1940 and Rules 1945 thereunder. IP is recognized as the official book of standards for the drugs being manufactured and/or marketed in India. IP contains a collection of authoritative procedures of analysis and specifications of drugs for their identity, purity and strength. The standards of the IP are authoritative in nature and are enforced by the regulatory authorities for ensuring the quality of drugs in India. During quality assurance and at the time of dispute in the court of law the IP standards are legally acceptable. The Standards prescribed in the IP are to establish compliance with regulatory requirements on an article. The criteria to be adhered to are: The interpretation of a monograph must be in accordance with all the general requirements, testing methods, texts, and notices pertaining to it, in the IP. A product is not of standard quality unless it complies with all the requirements of the monograph.
|
India Suriname Relations in detail:
https://www.iasgyan.in/daily-current-affairs/india-suriname-relations
https://www.iasgyan.in/daily-current-affairs/india-suriname-relations-12
Suriname
Location
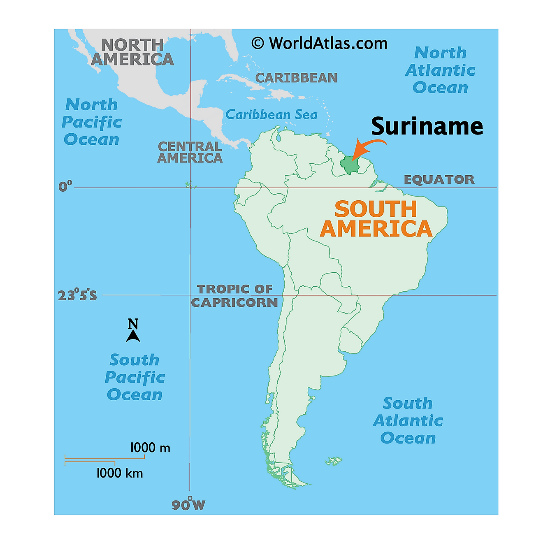
- Suriname is the smallest independent country in South America situated on the Guiana Shield.
Borders
- Suriname is situated between French Guiana to the east and Guyana to the west.
- The southern border is shared with Brazil and the northern border is the Atlantic coast.
Geographic Regions
- The country can be divided into two main geographic regions.
-
- The northern, lowland coastal area (roughly above the line Albina-Paranam-Wageningen) has been cultivated, and most of the population lives here.
- The southern part consists of tropical rainforest and sparsely inhabited savanna along the border with Brazil, covering about 80% of Suriname's land surface.
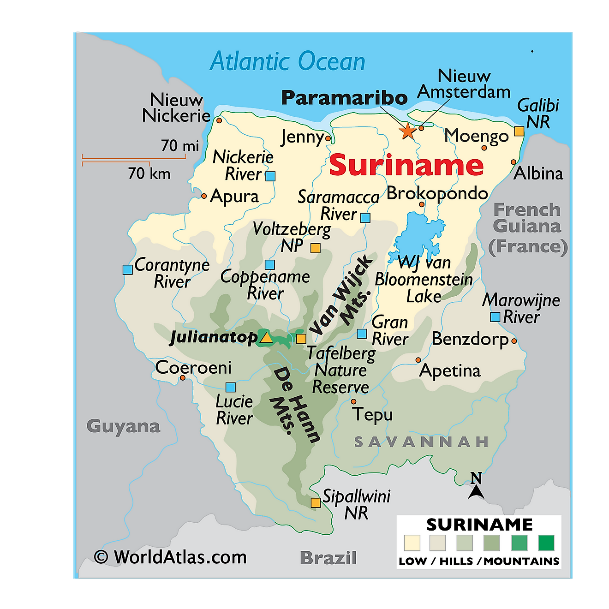
Mountain Ranges
- The two main mountain ranges are the Bakhuys Mountains and the Van Asch Van Wijck Mountains.
- Julianatop is the highest mountain in the country at 1,286 metres (4,219 ft) above sea level.
Ecoregions
- Suriname contains six terrestrial ecoregions: Guayanan Highlands moist forests, Guianan moist forests, Paramaribo swamp forests, Tepuis, Guianan savanna, and Guianan mangroves.
- Its forest cover is 90.2%, the highest of any nation in the world.
- The country had a 2019 Forest Landscape Integrity Index mean score of 9.39/10, ranking it fifth globally out of 172 countries.
Climate
- Lying two to five degrees north of the equator, Suriname has a very hot and wet tropical climate, and temperatures do not vary much throughout the year.
- The average relative humidity is between 80% and 90%.
Biodiversity and Conservation
- Snakewood (Brosimum guianense), a tree, is native to this tropical region of the Americas that is often illegally exported to French Guiana, for the crafts industry.
- The Central Suriname Nature Reserve has been designated a UNESCO World Heritage Site for its unspoiled forests and biodiversity.
|
PRACTICE QUESTION
Q. Consider the following statements:
1. Suriname is the smallest independent country in African Continent situated on the Guiana Shield.
2. Julianatop is the highest mountain in the country.
3. Its forest cover is 90.2%, the highest of any nation in the world.
How many of the above are correctly matched?
A) Only 1
B) Only 2
C) All 3
D) None
Answer: B) Only 2
|

https://pib.gov.in/PressReleasePage.aspx?PRID=1949414














