Description
Source: Hindustan Times
Disclaimer: Copyright infringement not intended.
Context
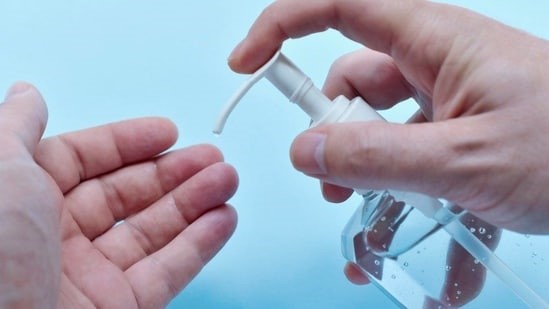
- Health officials in the United States have recalled several lots of hand sanitisers and aloe gels over the risk of methanol exposure.
Details
- The Food and Drug Administration recently announced that 40 lots of Aruba Aloe Hand Sanitizer Gel Alcohol 80% and Aruba Aloe Alcoholada Gel were recalled as they contain “alcohol denatured with methanol.”
- The FDA said in a notice that methanol can be highly toxic.
What is Methanol?
- Methanol, also known as methyl alcohol or wood alcohol, is a simple chemical compound with the formula CH₃OH.
- It is the simplest alcohol, consisting of a methyl group (-CH₃) linked to a hydroxyl group (-OH).
- Methanol is a colorless, volatile, flammable liquid with a characteristic odor.
- It is miscible with water and many organic solvents.
Properties of Methanol:
- Physical Properties: Methanol is a polar liquid at room temperature, with a boiling point of 64.7°C and a melting point of -97.6°C.
- Chemical Properties: It is a primary alcohol, undergoing typical alcohol reactions such as oxidation to form formaldehyde and further oxidation to form formic acid.
Uses of Methanol:
- Fuel: Methanol is used as a fuel or fuel additive, particularly in racing cars, where it is known as "methanol fuel" or "racing fuel." It is also being explored as a potential alternative fuel for internal combustion engines and fuel cells.
- Chemical Synthesis: Methanol serves as a key building block in the synthesis of numerous chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether (MTBE), and dimethyl ether (DME).
- Solvent: Due to its polar nature, methanol is used as a solvent in various industrial processes, such as in the production of resins, paints, and pharmaceuticals.
- Antifreeze: Methanol is sometimes used as an antifreeze in windshield washer fluid and automotive cooling systems, although its use is declining due to safety concerns.
- Biodiesel Production: Methanol is employed in the transesterification process to produce biodiesel from vegetable oils or animal fats.

Production Methods:
- Natural Sources: Methanol can be produced naturally in small quantities through the anaerobic metabolism of certain bacteria, as well as in the decomposition of organic matter.
- Synthetic Production: The primary method for commercial methanol production is through the catalytic conversion of carbon monoxide and hydrogen, known as the synthesis gas or syngas process. This process typically utilizes natural gas, coal, or biomass as the source of carbon monoxide and hydrogen.
Safety Considerations:
- Flammability: Methanol is highly flammable and should be handled with caution. Its vapors can form explosive mixtures with air.
- Toxicity: Methanol is toxic if ingested, inhaled, or absorbed through the skin. It metabolizes into formaldehyde and formic acid in the body, leading to potential organ damage, blindness, and even death.
- Ventilation: Adequate ventilation is crucial when working with methanol to prevent the buildup of vapors, which can pose health and safety risks.
- Storage: Methanol should be stored in tightly sealed containers away from heat, sparks, or open flames to prevent the risk of fire.
Environmental Impact:
- Greenhouse Gas Emissions: While methanol itself burns more cleanly than gasoline, its production process may contribute to greenhouse gas emissions, depending on the source of feedstock used.
- Water Pollution: Spills of methanol can contaminate water sources, posing risks to aquatic life and ecosystems.
- Renewable Methanol: Efforts are underway to produce methanol from renewable sources, such as biomass or carbon dioxide captured from industrial processes or the atmosphere, to reduce its environmental impact.

Conclusion
Methanol is a versatile chemical with numerous industrial applications, ranging from fuel and solvent to chemical synthesis. However, its flammability, toxicity, and environmental impact necessitate careful handling and management throughout its lifecycle, from production to use and disposal.
Sources:
Hindustan Times
|
PRACTICE QUESTION
Q. Methanol is a versatile chemical with numerous industrial applications, ranging from fuel and solvent to chemical synthesis. Discuss. (150 Words)
|
















