Description
Disclaimer: Copyright infringement not intended.
Context
- India has reached out to Australia seeking to restock monoclonal antibody doses to combat the Nipah virus.
Details
- The mortality among the infected is very high in Nipah — between 40% and 70% — compared to the mortality in COVID, which was 2% to 3%
- The monoclonal antibody being used to combat Nipah has passed the phase-one trial and has been administered to 14 individuals globally. None of the 14 individuals who received the antibody have died from the virus.
- The decision to use monoclonal antibodies depends on the state government, the patient, and the treating physician.
- The monoclonal antibody was developed in the United States and shared with an Australian university as part of a tech-transfer initiative.
- India received some doses of monoclonal antibodies from Australia in 2018.
- The monoclonal antibody is not an authorized treatment for Nipah, and there is no established treatment for the virus.
- Its safety is confirmed, but its effectiveness remains uncertain. It must be administered in the early stage of the infection.

Introduction to Monoclonal Antibodies
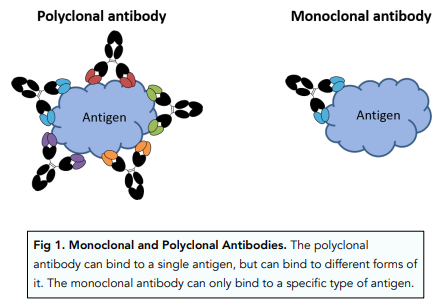
Definition and Basic Concept: Monoclonal antibodies (mAbs) are laboratory-produced molecules designed to mimic the immune system's ability to fight off harmful pathogens, such as bacteria and viruses. They are highly specific, targeting a single antigen or protein, and are used in various medical applications.
Historical Development and Significance: The development of monoclonal antibodies dates back to the 1970s when scientists first devised hybridoma technology, a method to create mAbs. The significance lies in their precision and versatility, making them invaluable tools in both diagnostics and therapies.
How Monoclonal Antibodies Work:
- Mechanism of Action: mAbs work by specifically binding to a target antigen, which can be a protein on the surface of a cancer cell, a virus, or an immune checkpoint molecule. This binding can lead to various outcomes, such as blocking the function of the antigen, flagging the target for destruction by the immune system, or delivering a drug payload to the target.
- Specificity and Selectivity: Monoclonal antibodies exhibit an extraordinary level of specificity. They are engineered to recognize and interact with a particular target, reducing the risk of off-target effects compared to conventional drugs that may affect multiple processes in the body.
- Comparison with Traditional Drugs: Compared to traditional drugs that often have broader effects, mAbs offer a more targeted approach. This specificity can lead to enhanced therapeutic efficacy and reduced side effects, though they can also be more expensive to develop and produce.
Production of Monoclonal Antibodies:
- Hybridoma Technology: Hybridoma technology involves fusing a specific antibody-producing B cell with a myeloma cell to create immortalized hybrid cells that continuously produce a single type of antibody. This method was the basis for the first monoclonal antibodies and is still used today.
- Recombinant DNA Technology: Recombinant DNA technology allows the production of mAbs in cell lines derived from animals or even humans. This approach can create antibodies with reduced immunogenicity, as they closely resemble human antibodies.
- Phage Display Technology: Phage display is an alternative method that utilizes bacteriophages to display antibodies on their surface. This technique enables the selection of antibodies with specific binding properties.
Types of Monoclonal Antibodies:
- Murine (Mouse-Derived) Antibodies: These antibodies are entirely derived from mice and can elicit an immune response when administered to humans, limiting their clinical use.
- Chimeric Antibodies: Chimeric antibodies combine mouse-derived antigen-binding regions with human constant regions, reducing immunogenicity.
- Humanized Antibodies: Humanized antibodies have the majority of their structure derived from human components, with only the antigen-binding region from mice.
- Fully Human Antibodies: Fully human antibodies are entirely derived from human sources and have minimal immunogenicity, making them suitable for therapeutic use.
Applications of Monoclonal Antibodies:
- Cancer Therapy: Monoclonal antibodies play a pivotal role in targeted cancer therapies. They can be designed to recognize specific antigens on cancer cells, hindering their growth, signaling the immune system to attack, or delivering toxic payloads directly to cancer cells.
- Autoimmune Diseases: In the treatment of autoimmune disorders, mAbs help modulate the immune system's response, reducing inflammation and damage to healthy tissues.
- Infectious Diseases: Monoclonal antibodies have applications in treating viral and bacterial infections. They can neutralize pathogens or inhibit their entry into host cells.
- Neurological Disorders: While less common, mAbs are being explored as potential treatments for neurological diseases, including Alzheimer's and multiple sclerosis.
Monoclonal Antibodies in Diagnosis:
- ELISA (Enzyme-Linked Immunosorbent Assay): Monoclonal antibodies are crucial components of ELISA tests used for the detection of antigens or antibodies in various diagnostic applications.
- Flow Cytometry: In flow cytometry, mAbs are used to label specific cell populations for analysis, aiding in disease diagnosis and research.
- Immunohistochemistry: Monoclonal antibodies are applied to tissue samples to identify specific proteins or antigens, aiding in the diagnosis of diseases such as cancer.
Challenges and Limitations:
- Immunogenicity: Some mAbs, especially those with non-human components, can provoke immune responses in patients, potentially reducing their effectiveness or causing adverse reactions.
- High Production Costs: The development and production of mAbs can be expensive, limiting their accessibility to patients, particularly in resource-constrained healthcare systems.
- Limited Penetration into Certain Tissues: Monoclonal antibodies may have difficulty penetrating certain tissues or crossing the blood-brain barrier, which can limit their effectiveness in treating diseases that affect these areas.
Future Trends and Developments:
- Advancements in Antibody Engineering: Ongoing research continues to refine antibody engineering techniques, enhancing their specificity and reducing immunogenicity.
- Personalized Medicine: The use of mAbs is aligning with the concept of personalized medicine, tailoring treatments to individual patients based on their unique biology.
- New Therapeutic Targets: As our understanding of disease mechanisms improves, novel therapeutic targets are emerging, and mAbs are likely to play a critical role in these treatments.
Economic and Ethical Considerations:
- Cost-Effectiveness of Monoclonal Antibodies: Assessing the cost-effectiveness of mAbs is crucial, as their high production costs can impact healthcare budgets and patient access.
- Access and Affordability: Ensuring equitable access to mAb therapies is an ethical concern, as these innovative treatments should be available to those who need them, regardless of their economic status.
- Ethical Issues: The use of animals in antibody production raises ethical questions. Efforts are made to minimize animal use and explore alternative methods.
|
PRACTICE QUESTION
Q. Explain the significance of monoclonal antibodies in modern medicine. Discuss their applications, challenges, and the ethical considerations associated with their production and use. (250 Words)
|
.jpg)
https://www.thehindu.com/sci-tech/health/india-to-procure-20-more-doses-of-monoclonal-antibody-from-australia-for-nipah-treatment-says-icmr-dg/article67311437.ece