Description
Context: Indigenous mRNA vaccine candidate supported by DBT gets Drug Controller nod to initiate Human clinical trials.
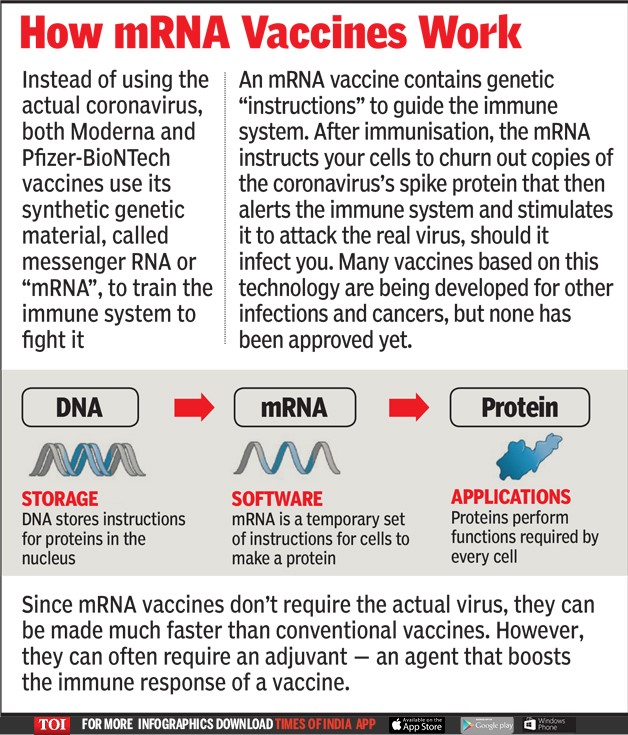
- The mRNA vaccines do not use the conventional model to produce immune response.
- Instead, mRNA vaccine carries the molecular instructions to make the protein in the body through a synthetic RNA of the virus.
- The host body uses this to produce the viral protein that is recognized and thereby making the body mount an immune response against the disease.
- mRNA-based vaccines are scientifically the ideal choice to address a pandemic because of their rapid developmental timeline.
- The mRNA vaccine is considered safe as is non-infectious, non-integrating in nature, and degraded by standard cellular mechanisms.
- They are expected to be highly efficacious because of their inherent capability of being translatable into the protein structure inside the cell cytoplasm.
- Additionally, mRNA vaccines are fully synthetic and do not require a host for growth, e.g., eggs or bacteria. Therefore, they can be quickly manufactured in an inexpensive manner under cGMP conditions to ensure their "availability" and "accessibility" for mass vaccination on a sustainable basis.
Ind-CEPI mission
- The Department of Biotechnology, Ministry of Science & Technology, Government of India is implementing the Ind-CEPI mission ‘India Centric Epidemic Preparedness through Rapid Vaccine Development: Supporting Indian Vaccine Development’ which is aligned with the Global Initiative of CEPIand aims to strengthen the development of vaccines and associated competencies/technologies for the diseases of epidemic potential in India.
- The Ind-CEPI mission of DBT is implemented by its PSU, Biotechnology Industry Research Assistance Council (BIRAC).
https://www.pib.gov.in/PressReleseDetail.aspx?PRID=1680031















