





Disclaimer: Copyright infringement not intended.
Context
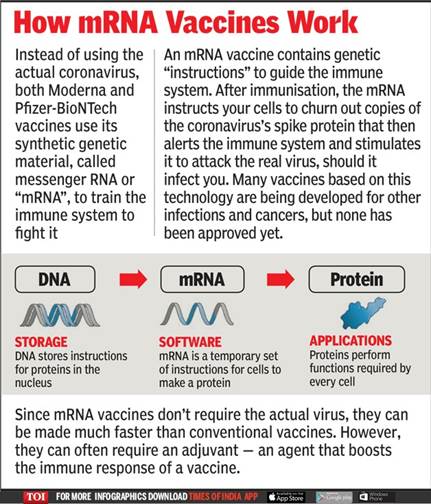
mRNA vaccines
https://www.thehindu.com/news/national/dcgi-nod-restricted-emergency-use-indigenous-mrna-covid-jab-18-years-above/article65577382.ece






© 2026 iasgyan. All right reserved