Description

Copyright infringement not intended
Context: The GST Council, in its 50th meeting, announced some important exemptions on IGST for certain medical imports, intending to enhance the availability of vital medical treatments and support the healthcare institutions that cater to rare diseases.
Details
- The 50th GST Council recommended exempting Dinutuximab (Qarziba), a rare cancer drug, as well as medicines and Food for Special Medical Purposes (FSMP) used in the treatment of rare diseases listed under the National Policy for Rare Diseases, 2021, from IGST when imported for personal use, subject to certain conditions.
- Additionally, imports of medicines and FSMP used in the treatment of rare diseases were also exempted from Integrated GST (IGST). Earlier, such imports were subject to an IGST of either 5% or 12%.
- Industry experts highlighted the significance of extending Customs and GST waiver benefits to all life-saving medicines, including cancer medicines that are imported. The experts contended that this would enable patients in India to avail of duty waiver benefits and reduce their treatment costs.

Rare Diseases and the National Policy for Rare Diseases 2021
About
- Rare diseases (also called “Orphan” diseases) are health conditions that affect a small number of people compared to other prevalent diseases in the general population. They are often genetic, chronic, and life-threatening, and can cause significant physical and mental disabilities.
- There is no universally accepted definition of a rare disease, but different countries have adopted different criteria based on prevalence, severity, and availability of treatment. In India, a rare disease is defined as one that affects less than 1 in 10,000 people.
- According to some estimates, there are about 6,000 to 8,000 rare diseases in the world, affecting about 6% to 8% of the population. However, 80% of rare disease patients are affected by approximately 350 rare diseases. Some of the common rare diseases affecting people in India are haemophilia, sickle cell anaemia, thalassemia, etc.
- The National Policy for Rare Diseases 2021 aims to provide a comprehensive framework for the prevention, control, screening, diagnosis, management, and treatment of rare diseases in India. The policy was formulated after extensive consultations with various stakeholders, including experts, patient associations, civil society organizations, state governments, and international agencies.
The National Policy for Rare Diseases policy covers the following aspects:
Definition and classification of rare diseases
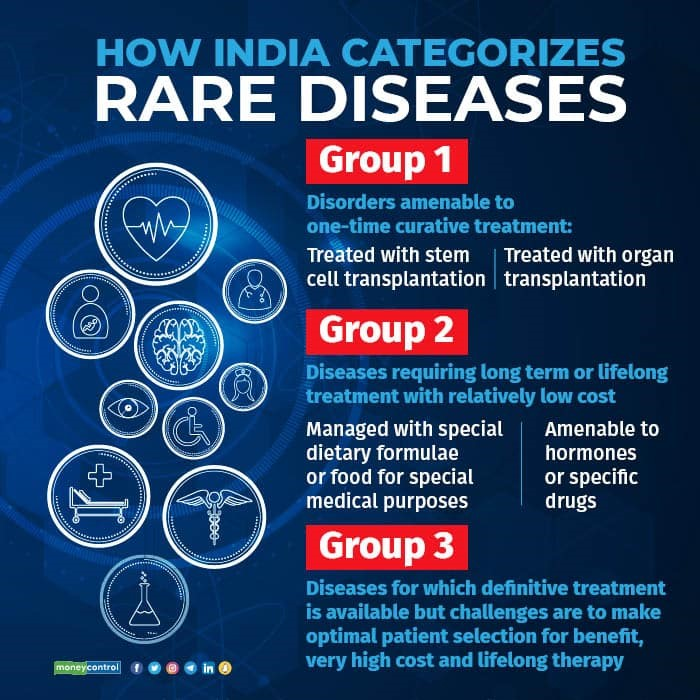
- The policy defines a rare disease and also classifies rare diseases into three groups based on the availability and feasibility of treatment:
- Group 1 includes disorders that are amenable to one-time curative treatment such as bone marrow transplantation or gene therapy.
- Group 2 includes disorders that require long-term or lifelong treatment such as enzyme replacement therapy or hormonal therapy.
- Group 3 includes disorders that have no definitive treatment but can be managed with supportive care or symptomatic treatment.
Prevention and Control
- The policy emphasizes the need for the prevention and control of rare diseases through various measures such as prenatal screening and testing, newborn screening, carrier testing, premarital counselling, genetic counselling, and public awareness campaigns.
- The policy also proposes to establish a national registry for rare diseases to collect data on the prevalence, incidence, morbidity, mortality, and outcomes of rare diseases in India.
Centres of excellence and NIDAN Kendras
- The policy proposes to designate certain tertiary care hospitals as centres of excellence for rare diseases. It will provide comprehensive care for rare disease patients including diagnosis, management, treatment, counselling, and follow-up. It will conduct research and training on rare diseases and collaborate with national and international agencies.
- The policy also proposes to strengthen the existing network of NIDAN Kendras (National Inherited Diseases Administration Kendras) which are diagnostic laboratories for genetic testing of rare diseases.
Government support in the treatment
- The policy proposes to provide financial support for the treatment of rare diseases under different schemes.
- For Group 1 disorders that require one-time curative treatment, the policy proposes to cover the cost of treatment under the Rashtriya Arogya Nidhi (RAN) scheme or any other scheme as per the state government's discretion.
- For Group 2 disorders that require long-term or lifelong treatment, the policy proposes to subsidize the cost of treatment under the Ayushman Bharat Pradhan Mantri Jan Arogya Yojana (AB-PMJAY) scheme or any other scheme as per the state government's discretion.
- For Group 3 disorders that have no definitive treatment, the policy proposes to provide supportive care and symptomatic treatment under various existing schemes such as the National Health Mission (NHM), the National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases and Stroke (NPCDCS), etc.
- The policy provides financial support of up to Rs 50 lakh for the treatment of rare diseases.
Development of manpower
- The policy proposes to develop human resources for rare diseases by creating a pool of trained professionals such as geneticists, genetic counsellors, laboratory technicians, nurses, social workers, etc. The policy also proposes to introduce courses on rare diseases in medical colleges and other health institutions.
Increasing affordability of drugs related to rare diseases
- The policy proposes to increase the affordability and accessibility of drugs related to rare diseases by various measures such as promoting indigenous production, encouraging voluntary licensing, facilitating import and export, reducing taxes and duties, and regulating prices.
Significances
- It provides a clear definition and classification of rare diseases which would help in identifying and prioritizing the patients who need urgent intervention.
- It provides financial support to patients with curable rare diseases which would improve their access to treatment and quality of life.
- It focuses on prevention and early detection of rare diseases which would reduce the morbidity and mortality associated with them.
- It strengthens the health system's capacity for the diagnosis and management of rare diseases through CoEs, Nidan Kendras, AB-HWCs etc.
- It promotes indigenous research and innovation in the field of rare diseases which would enhance the availability and affordability of drugs and devices for them.
- It fosters collaboration and coordination among various stakeholders such as government, academia, industry, civil society etc. for addressing the issues related to rare diseases.

Conclusion
- The National Policy for Rare Diseases 2021 is a landmark document that reflects the government's commitment and vision to address the challenges and needs of rare disease patients and their families in India. It is expected that the policy will provide a holistic and integrated approach to prevent, control, diagnose, manage, and treating rare diseases in India. It is also hoped that the policy will foster a culture of research, innovation, and collaboration for rare diseases in India and globally.
Keywords
National Health Mission
- It is a flagship initiative of the Indian government that started in 2013. It integrates the National Rural Health Mission (NRHM), which began in 2005, and the National Urban Health Mission (NUHM), which began in 2013.
- It aims to provide universal access to quality healthcare services that are fair, affordable and responsive to people's needs.
- It focuses on strengthening the health system in both rural and urban areas and addressing the health needs of - Reproductive-Maternal- Neonatal-Child and Adolescent Health (RMNCH+A), and Communicable and Non-Communicable Diseases.
IGST
- Integrated GST (IGST) is a mechanism that facilitates the collection and distribution of taxes on goods and services that are traded across state boundaries.
- It is not a separate tax, but a way of ensuring that the state and union governments receive their respective shares of the GST revenue.
- It is levied by the central government on inter-state transactions and then apportioned between the states based on the destination principle. This means that the tax revenue goes to the state where the goods or services are consumed, not where they are produced or supplied.
Ayushman Bharat Pradhan Mantri Jan Arogya Yojana
- Ayushman Bharat-PMJAY is a government-funded health insurance/ assurance scheme that covers over 10 crore poor and vulnerable families in India.
- It was launched in 2018 and provides a cover of Rs.5 lakh per family per year for secondary and tertiary care hospitalization.
- It is an entitlement-based scheme, which means that the beneficiaries are identified based on the latest Socio-Economic Caste Census (SECC) data and do not need to enrol or pay any premium.
- The scheme is implemented by the National Health Authority (NHA) at the central level and the State Health Agency (SHA) at the state level.
Must-Read Articles:
50th MEETING OF GST COUNCIL: https://www.iasgyan.in/daily-current-affairs/50th-meeting-of-gst-council
NATIONAL POLICY FOR RARE DISEASES 2021: https://www.iasgyan.in/daily-current-affairs/national-policy-for-rare-diseases-2021-7
|
PRACTICE QUESTION
Q. Which of the following statements is/are correct about the National Policy for Rare Diseases 2021?
1. It provides financial assistance for one-time treatments of up to Rs. 50 lakh for certain rare diseases.
2. It establishes a national hospital-based registry of rare diseases to collect data and facilitate research and development.
3. It sets up a National Consortium for Research and Development on therapeutics for rare diseases.
Which of the following Statement is/are correct?
(A) 1 and 2 only
(B) 2 and 3 only
(C) 1 and 3 only
(D) 1, 2 and 3
Answer: D
Explanation: The National Policy for Rare Diseases 2021 aims to lower the high cost of treatment for rare diseases with an increased focus on indigenous research. It provides financial assistance for one-time treatments of up to Rs. 50 lakh for certain rare diseases, establishes a national hospital-based registry of rare diseases to collect data and facilitate research and development and sets up a National Consortium for Research and Development on therapeutics for rare diseases.
|
https://www.business-standard.com/economy/news/gst-council-s-waiver-for-rare-diseases-drugs-little-help-for-patients-123071200845_1.html


















