Description

Disclaimer: Copyright infringement not intended.
Context
- According to Central Pollution Control Board (CPCB) bulletins, ozone was identified as a prominent pollutant in Delhi.
|
DID YOU KNOW?
The hourly ozone level standard is 180 µg/m3 as set by National Ambient Air Quality Standards.
|
What is Ozone?
- Ozone (O3) is a molecule made up of three atoms of oxygen (O), and is mostly found in the stratosphere, where it protects us from the Sun’s harmful ultraviolet (UV) radiation.
- Although it represents only a tiny fraction of the atmosphere, ozone is crucial for life on Earth.
- Ozone in the stratosphere—a layer of the atmosphere between 15 and 50 kilometers (10 and 31 miles) above us—acts as a shield to protect Earth’s surface from the sun’s harmful ultraviolet radiation.
- Without ozone, the Sun’s intense UV radiation would sterilize the Earth’s surface.
- With a weakening of this shield, more intense UV-B and UV-A radiation exposure at the surface would lead to quicker sunburns, skin cancer, and even reduced crop yields in plants.
- However, near the surface where we live and breathe, ozone is a harmful pollutant that causes damage to lung tissue and plants.
- This “bad” ozone forms when sunlight initiates chemical reactions in the air involving pollutants, particularly a family of gases called nitrogen oxides (released from vehicles and industry during the combustion process) and with volatile organic compounds (carbon-containing chemicals that evaporate easily into the air, such as petroleum products).
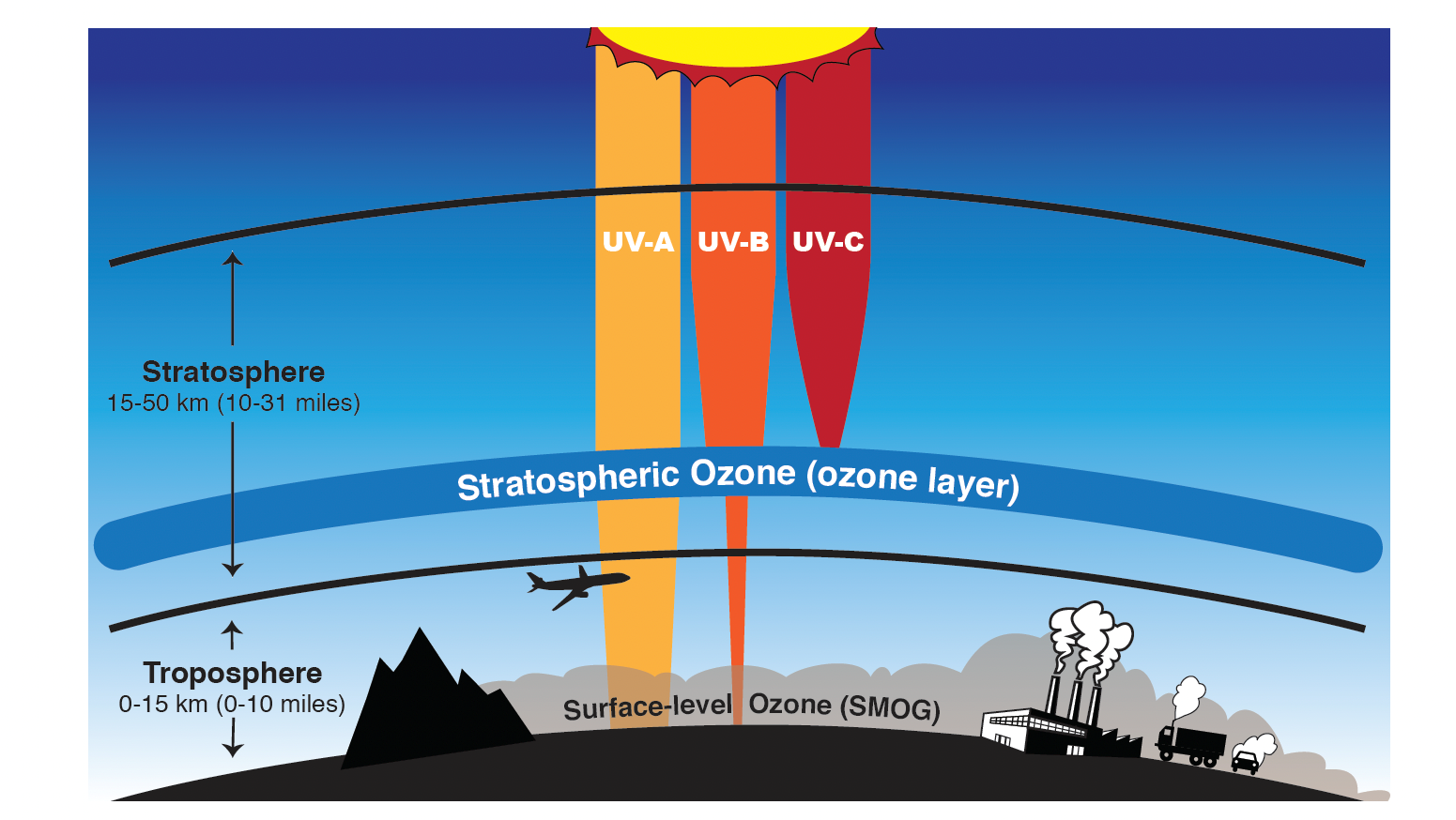
Ozone is good in the stratosphere because it absorbs all of the most energetic ultraviolet radiation (UV-C), most of the UV-B radiation and some of the least energetic UV radiation (UV-A). Ozone is “bad” in the troposphere because it is harmful to breathe and is the primary component of smog in summer.
Chemistry of the Ozone Layer
- There are natural processes that create and destroy ozone in the stratosphere. These processes regulate a balance of ozone and form the ozone layer.
- Ozone is created primarily by sunlight.
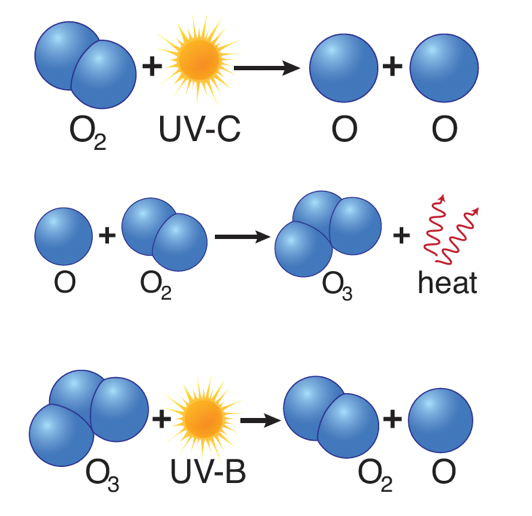
- When high-energy ultraviolet rays (UV-C) strike an oxygen molecule (O2), they split the molecule into two single oxygen atoms, known as atomic oxygen.
- A freed oxygen atom then combines with another oxygen molecule to form a molecule of ozone (O3).
- Because there is so much oxygen in our atmosphere, this “ozone-oxygen cycle” is continuously absorbing high-energy ultraviolet radiation (UV-C) and completely blocking it from reaching the Earth’s surface.
- This process creates heat which warms the upper part of the stratosphere.
- Ozone is very reactive, and attacks other molecules in the air, often regenerating oxygen in the process.
- Also—and this is why ozone is important to us—ozone in the stratosphere absorbs much of the sun’s UV-B rays, splitting back into molecular and atomic oxygen.
- No matter how the oxygen atoms are produced, they almost always quickly react with oxygen molecules, reforming ozone.
- So, while ozone is continually being replenished, it is also continually being destroyed.
- Sometimes an ozone molecule reacts with an oxygen atom, creating two oxygen molecules, thus ending the cycle.
- If the rate of ozone creation is equal to the rate of destruction, the total amount will remain the same.
- Scientists have found that ozone levels change periodically with regular natural cycles such as the changing seasons, winds, and long time scale sun variations.
- Ozone also responds to some sporadic solar events such as flares. Moreover, volcanic eruptions may inject materials into the stratosphere that can lead to increased destruction of ozone.
- In the 1970’s, scientists suspected that reactions involving man-made chlorine-containing compounds could upset this balance leading to lower levels of ozone in the stratosphere.
- Human production of chlorine-containing chemicals, such as chlorofluorocarbons (CFCs), has added an additional factor that destroys ozone.
- CFCs are molecules made up of chlorine, fluorine and carbon. Because they are extremely stable molecules, CFCs do not react with other chemicals in the lower atmosphere, but exposure to ultraviolet radiation in the stratosphere breaks them apart, releasing chlorine atoms.
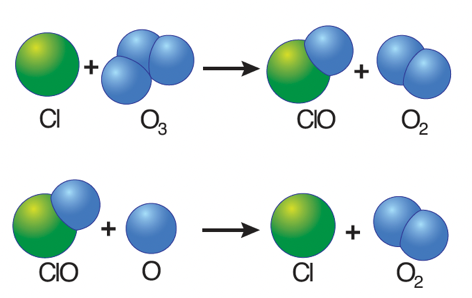
- Free chlorine (Cl) atoms then react with ozone molecules, taking one oxygen atom to form chlorine monoxide (ClO) and leaving an oxygen molecule (O2).
- If each chlorine atom released from a CFC molecule destroyed only one ozone molecule, CFCs would pose very little threat to the ozone layer.
- However, when a chlorine monoxide molecule encounters a free atom of oxygen, the oxygen atom breaks up the chlorine monoxide, stealing the oxygen atom and releasing the chlorine atom back into the stratosphere to destroy another ozone molecule.
- These two reactions happen over and over again, so that a single atom of chlorine, acting as a catalyst, destroys many molecules (about 100,000) of ozone.
- Fortunately, chlorine atoms do not remain in the stratosphere forever. Free chlorine atoms react with gases, such as methane (CH4), and get bound up into hydrogen chloride (HCl) molecules
- These molecules eventually end up back in the troposphere where they are washed away by rain.
- Therefore, if humans stop putting CFCs and other ozone-destroying chemicals into the stratosphere, stratospheric ozone will eventually return to its earlier, higher values.

How the Ozone Hole forms?
- The Ozone Hole is not really a “hole” but a thinning of the ozone layer over the south polar region.
- Every year, since at least 1978, there is a sudden, rapid decrease in the stratospheric ozone levels at the end of the Antarctic winter.
- During the long winter months of darkness over the Antarctic, atmospheric temperatures drop, creating unique conditions for chemical reactions that are not found anywhere else in the atmosphere.
- The wind in the stratosphere over the polar region intensifies and forms a polar vortex, which circulates around the pole. The transition from inside to outside the polar vortex creates a wind barrier that isolates the air inside the vortex and also results in very cold temperatures.
- At temperatures below -78°C, thin clouds made of mixtures of ice, nitric acid, and sulfuric acid form in the stratosphere.
- Chemical reactions on the surfaces of these ice crystals convert chlorine-containing compounds like HCl, which is harmless to ozone, into more reactive forms.
- When the sun rises over the Antarctic in the Spring (September), light rapidly releases free chlorine atoms into the stratosphere. A new ozone destroying cycle begins.
- The chlorine atoms react with ozone, creating ClO. The ClO molecules combine with each other, forming a compound called a dimer.
- Sunlight releases chlorine atoms from the dimer, and the cycle begins again. The polar vortex keeps the ozone-depleted air inside from mixing with the undepleted air outside the vortex.
- The ozone destruction continues within the polar vortex until the ozone levels approaches zero at the altitudes where reactions on the thin clouds have released chlorine atoms.
- Once ozone has reached such a low level, the chlorine atoms react with methane, filling the vortex with HCl.
- The low ozone persists until the vortex weakens and breaks apart.
- Then the ozone levels in the polar stratosphere begin to return to pre-September levels due to the increase in solar UV and the mixing of polar and nonpolar air.
When was the problem discovered?
- In 1974, chemists Mario Molina and Frank Sherwood Rowland discovered a link between chlorofluorocarbons (CFCs) and the breakdown of ozone in the stratosphere.
- In 1985, geophysicist Joe Farman, along with meteorologists Brian G Gardiner and Jon Shanklin published findings of abnormally low ozone concentrations above the Antarctic.
Timeline
- 1974: Chemists in the USA discover the link between CFCs and the breakdown of ozone in the stratosphere
- 1985: British scientists publish results of abnormally low ozone concentrations above the Antarctic
- 1985: Vienna Convention for the Protection of the Ozone Layer agreed
- 1987: Montreal Protocol on Substances that Deplete the Ozone Layer agreed
- 1989: Ozone Protection and Synthetic Greenhouse Gas Management Act commenced in Australia
- 1991: Phase out of CFCs (chlorofluorocarbons) begins
- 1996: Phase out of HCFCs (hydrochlorofluorocarbons) begins
- 2016: Kigali Amendment to Montreal Protocol (aims for the phase-down of hydrofluorocarbons (HFCs) by cutting their production and consumption).

‘Scientific Assessment of Ozone Depletion: 2018’: a UN Study
- It has shown that the ozone layer is recovering at a rate of 1-3% per decade.
World Ozone Day
- It is celebrated every year on 16 September.
|
PRACTICE QUESTION
Q) While ozone is continually being replenished, it is also continually being destroyed. Discuss in brief the scientific phenomenon associated with this statement. (150 words)
|
https://indianexpress.com/article/cities/delhi/why-ozone-prominent-pollutant-over-past-two-days-in-delhi-8595685/















