Description
Disclaimer: Copyright infringement not intended.
Context
- Researchers at the Indian Institute of Science Education and Research (IISER), Bhopal, have pioneered a groundbreaking advancement in photocatalysis by creating a potent and sustainable material named UC-POP-Au.
Details
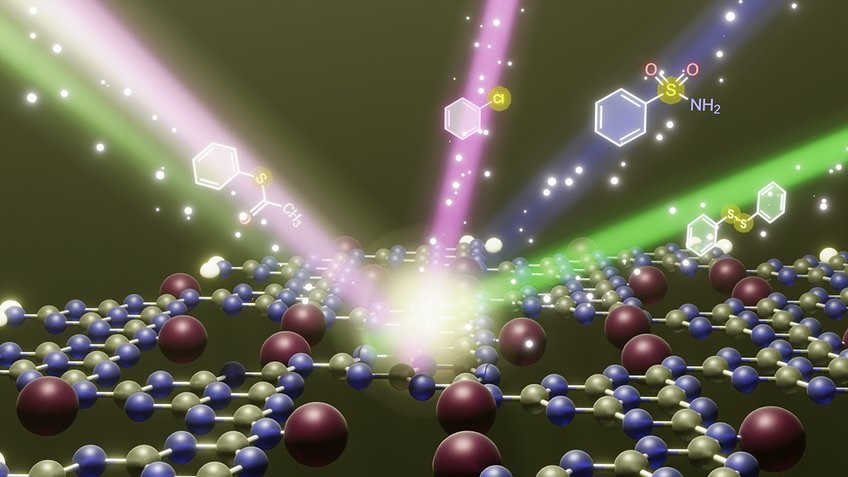
- This novel photocatalyst, combining upconversion nanoparticles and gold nanoparticles within a UV-absorbing porous organic polymer, exhibits exceptional capabilities in harnessing solar energy for chemical processes, specifically targeting the detoxification of hazardous substances like chemical warfare agents.
UC-POP-Au Photocatalyst
- Full Spectrum Light Absorption: UC-POP-Au demonstrates a remarkable ability to absorb the entire spectrum of light, unlike traditional photocatalysts that only utilize UV or high-energy light segments. This property significantly enhances its catalytic efficiency in chemical reactions under sunlight.
- Detoxification of Mustard Gas Simulant: The material has displayed impressive efficiency in detoxifying mustard gas simulants, such as '2-chloroethyl ethyl sulfide' (CEES), a highly toxic chemical warfare agent. Under direct sunlight, UC-POP-Au exhibited superior performance in breaking down CEES, showcasing its potential in combating chemical threats.
- Composition and Application: The composition of UC-POP-Au, integrating near-infrared absorbing upconversion nanoparticles and visible light absorbing gold nanoparticles within a UV-absorbing porous organic polymer, contributes to its catalytic efficacy.
- Real-World Applications: in designing protective coatings against chemical warfare agents under natural sunlight conditions. Its successful utilization on cotton cloth to detoxify mustard gas simulants exemplifies its practicality.
- Reusability and Sustainability: UC-POP-Au demonstrates reusability, retaining its catalytic activity over multiple cycles. This sustainability aspect distinguishes it from other catalysts that lack the ability to be collected and reused.

Introduction to Photocatalysis
- Photocatalysis involves a process where light energy triggers a chemical reaction by activating a photocatalyst.
- These catalysts play a pivotal role in accelerating chemical transformations under light irradiation without undergoing chemical changes themselves.
Working Principles:
- Photocatalyst Function: Photocatalysts operate by absorbing photons from light, subsequently initiating reactions by exciting electrons within the catalyst's structure.
- Generation of Electron-Hole Pairs: Light absorption promotes electrons to higher energy levels, creating electron-hole pairs. These charged species drive chemical transformations by participating in redox reactions.
- Surface Reactions: Electrons and holes participate in surface reactions, facilitating the degradation of pollutants, water splitting for hydrogen production, and organic synthesis.
Types of Photocatalysts:
- Metal Oxides: Titanium dioxide (TiO2) and zinc oxide (ZnO) are extensively studied metal oxide photocatalysts due to their stability, abundance, and efficiency in various photocatalytic applications.
- Semiconductors: Certain semiconductors, like cadmium sulfide (CdS) and tungsten trioxide (WO3), exhibit photocatalytic properties owing to their band structures conducive to light absorption and charge separation.
- Organic Photocatalysts: Organic molecules, particularly organic dyes and porphyrins, demonstrate photocatalytic activity, primarily in organic synthesis and pollutant degradation.
Applications of Photocatalysts:
- Environmental Remediation: Photocatalysts assist in degrading organic pollutants, purifying water, and reducing air pollutants, contributing to environmental sustainability.
- Hydrogen Production: Photocatalysts enable the conversion of water into hydrogen and oxygen through water splitting, a clean energy source for fuel cells and energy storage.
- Solar Energy Conversion: Photocatalytic systems aid in harnessing solar energy for various applications, including solar cells and artificial photosynthesis.
- Chemical Synthesis: Utilization of photocatalysts in synthetic chemistry facilitates the synthesis of fine chemicals and pharmaceuticals with enhanced efficiency and selectivity.
Introduction to Chemical Warfare Agents
- Chemical warfare agents (CWAs) are toxic chemicals primarily designed for military use to incapacitate, injure, or kill humans through exposure via various routes, including inhalation, ingestion, or skin contact.
- These substances are classified based on their chemical properties and effects on the human body.
Classification of Chemical Warfare Agents:
- Nerve Agents: Highly toxic organophosphorus compounds disrupting the nervous system. Examples include Sarin, Tabun, Soman, VX.
- Blister Agents (Vesicants): Chemicals causing severe skin, eye, and respiratory tract irritation and blistering. Mustard gas (Sulfur mustard) and Lewisite fall into this category.
- Blood Agents: Compounds disrupting oxygen utilization in the body. Hydrogen Cyanide (AC) and Cyanogen Chloride (CK) are notable examples.
- Choking Agents: Substances causing severe respiratory distress and lung damage. Chlorine and Phosgene are commonly known choking agents.
Effects on Human Health:
- Nerve Agents: Rapidly affect the nervous system, causing symptoms like convulsions, respiratory distress, and ultimately leading to paralysis and death.
- Blister Agents: Induce skin and eye irritation, leading to severe burns, blistering, and potential long-term health complications.
- Blood Agents: Interfere with oxygen transport in the body, causing rapid asphyxiation and death.
- Choking Agents: Severe damage to the respiratory system, including pulmonary edema, leading to suffocation and death.
Historical Use:
- World Wars: CWAs were extensively used in World War I and sporadically in conflicts thereafter, leading to widespread casualties and long-term health effects.
- Modern Warfare: Despite international treaties prohibiting their use, instances of CWAs being deployed in conflicts have occurred in recent history.
International Conventions and Treaties:
- Chemical Weapons Convention (CWC): An international treaty prohibiting the development, production, stockpiling, and use of CWAs, ensuring their destruction and promoting peaceful use of chemistry.
- Organizations and Oversight: The Organization for the Prohibition of Chemical Weapons (OPCW) oversees the implementation of the CWC and verifies compliance among member states.
Introduction to Mustard Gas
- Mustard gas, also known as Sulfur Mustard (HD - bis(2-chloroethyl) sulfide), is a potent and blistering chemical warfare agent that gained notoriety for its devastating effects during World War I and subsequent conflicts.
- It belongs to the category of vesicant agents used in chemical warfare due to its ability to cause severe blistering and tissue damage upon exposure.
Properties and Mechanism of Action:
- Chemical Composition: Mustard gas is a sulfur-based organic compound with a yellow-brown color and a distinctive garlic-like odor. It can be produced in liquid, vapor, or aerosol forms.
- Mode of Action: Mustard gas primarily affects the skin, eyes, and respiratory system upon contact. It interferes with DNA synthesis, causing cell death and severe blistering in affected areas.

Effects on Human Health:
- Skin Effects: Contact with mustard gas leads to skin irritation, blistering, and burns. Blisters typically appear within hours of exposure and can be extremely painful, causing long-term scarring and tissue damage.
- Respiratory Effects: Inhalation of mustard gas vapors causes irritation and damage to the respiratory tract, leading to coughing, shortness of breath, and potentially life-threatening lung damage.
- Eye Irritation: Exposure to mustard gas vapor causes eye irritation, redness, tearing, and in severe cases, blindness.
Conclusion
In summary, the groundbreaking UC-POP-Au photocatalyst developed by IISER Bhopal opens new dimensions in photocatalysis by harnessing sunlight to combat chemical threats, offering potential solutions for environmental and security challenges.
|
PRACTICE QUESTION
Q. Elaborate on the significance of the UC-POP-Au photocatalyst developed by IISER Bhopal researchers in the context of chemical warfare agent neutralization. (250 Words)
|















