Description
Disclaimer: Copyright infringement not intended.
Context
Archaeologists have used radiocarbon dating to analyze the oldest true wooden frame saddle in East Asia, revealing how the rise of Mongolian steppe cultures was likely aided by advances in equestrian technology.
Details
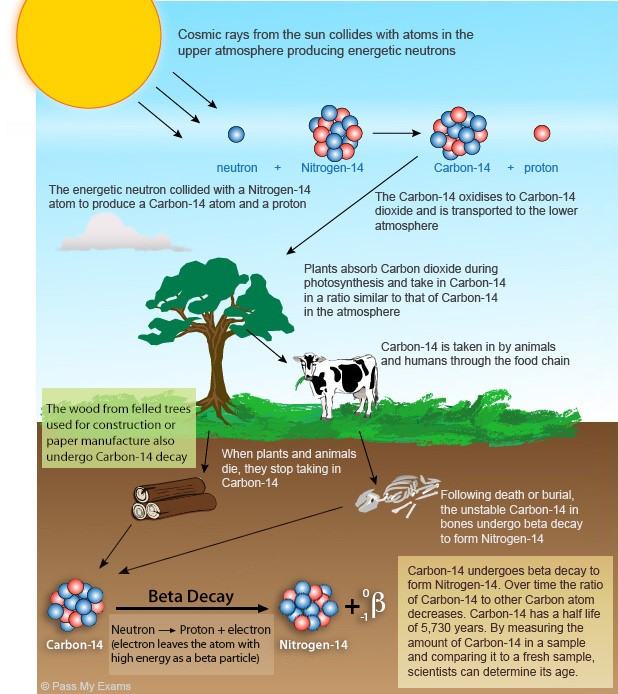
- Radiocarbon dating, also known as carbon-14 dating, is a widely used method for determining the age of organic materials based on the decay rate of the radioactive isotope carbon-14 (^14C).
- This technique has revolutionized archaeology, anthropology, and other fields that study the past.
What is Carbon-14?
- Carbon is an essential element found in all living organisms.
- Most carbon atoms are stable, but a small fraction are radioactive isotopes like carbon-14.
- Cosmic rays constantly bombard the Earth's atmosphere, producing ^14C.
- When living organisms consume carbon through photosynthesis or eating, they incorporate a small amount of carbon-14 into their tissues.

Radioactive Decay of Carbon-14:
- Carbon-14 is radioactive and undergoes a process called radioactive decay.
- It decays into nitrogen-14 (^14N) through beta decay, emitting a beta particle (electron) and an antineutrino in the process.
- The half-life of carbon-14 is approximately 5,730 years.
- This means that after this period, half of the initial amount of carbon-14 in a sample will have decayed to nitrogen-14.
Dating Process
- Sample Collection: Archaeologists or scientists extract samples containing organic material, such as wood, bone, charcoal, or organic sediments, from the site of interest.
- Isolation of Carbon: The extracted material undergoes processing to isolate carbon in the form of graphite or carbon dioxide gas.
- Measuring Carbon-14 Content: Accelerator Mass Spectrometry (AMS) or other sensitive techniques are used to measure the ratio of carbon-14 to stable carbon-12 (^12C) in the sample.
- Calibration: The obtained ratio is compared to the known standard ratio to correct for fluctuations in atmospheric carbon-14 levels throughout history. This calibration accounts for changes caused by factors like solar activity and volcanic eruptions.
- Calculating Age: Using the measured ratio of ^14C to ^12C and its known half-life, scientists calculate the age of the sample. The equation used is based on the exponential decay of carbon-14.
Limitations and Considerations
- Half-Life Limitation: Radiocarbon dating is effective up to around 50,000 years, as after this time, the amount of remaining carbon-14 becomes too low to accurately measure.
- Contamination: Contamination by newer or older carbon-containing substances can skew results. Careful sample preparation and analysis are crucial to avoid this.
- Calibration Issues: Calibration curves are used to account for fluctuations in atmospheric ^14C levels, but uncertainties can still exist, affecting the accuracy of the dating.
Applications
- Archaeology: Dating ancient artifacts, human remains, and historical sites.
- Geology: Studying the age of organic materials in geological contexts.
- Climate Science: Analyzing carbon cycles and changes in the Earth's atmosphere.


Conclusion
Radiocarbon dating provides invaluable insights into the age of organic materials and has significantly contributed to our understanding of human history and the natural world. Despite its limitations, it remains a powerful tool in scientific research. Continuous advancements in technology and calibration methods enhance its accuracy and broaden its applications across various disciplines.
|
PRACTICE QUESTION
Q. Discuss the significance of radiocarbon dating in determining the chronology of archaeological sites and its contribution to understanding human history. Highlight its limitations and the advancements in the field that have enhanced its accuracy. (250 Words)
|