Description
Disclaimer: Copyright infringement not intended.
Context
- The World Health Organisation (WHO) issued an alert over a batch of India-made common cold syrup sold in Iraq after it found the product "contaminated" and "unsafe" for consumption.
Details
- The syrup, branded Cold Out, was being sold in Iraq and manufactured by Fourrts (India) Laboratories for Dabilife Pharma.
- The global health agency discovered that the syrup had a higher than acceptable limit of contaminants -- diethylene and ethylene glycol.
- In its medical product alert, the WHO said the batch of the syrup had 0.25 per cent of diethylene glycol and 2.1 per cent of ethylene glycol, when the acceptable safety limit for both of them is up to 0.10 per cent.
- Diethylene glycol and ethylene glycol are toxic to humans when consumed and can prove fatal.
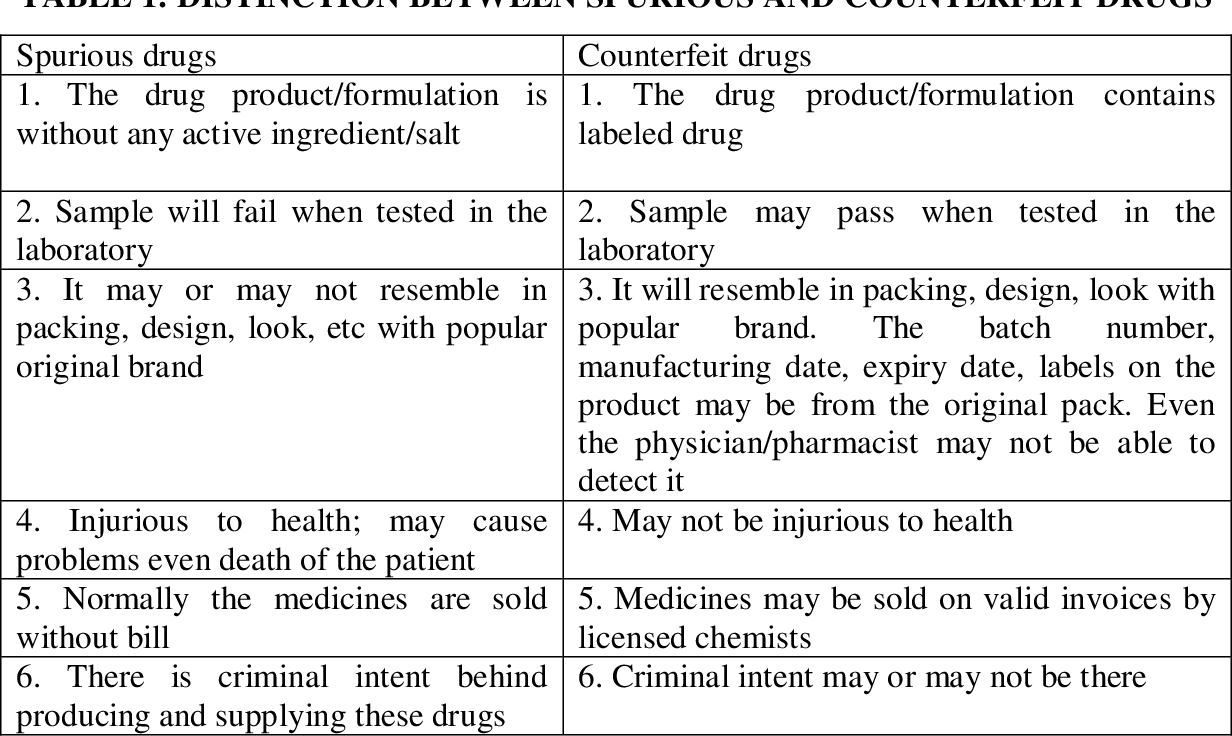
What are spurious and adulterated drugs?
- The Drugs and Cosmetics Act, 1940 defines a drug as spurious if it is manufactured under a name that belongs to another drug or if it is in imitation of or substitute of another drug and purports the purpose of a manufacturer of whom it is truly a product.
- A drug is deemed adulterated if it consists in whole or part any filthy, putrid or decomposed substance; if it is prepared, packed or stored under insanitary conditions; if it contains any harmful or toxic substance which may render it injurious to health; or if any substance has been mixed therewith so as to reduce its quality or strength, according to the Act.
- Substandard drugs work less effectively, causing the disease to run a longer course, and can even require a new prescription during treatment. They also contribute to antibacterial resistance, a threat that doubled in India between 2011 and 2016.
- The Drugs and Cosmetics Act 1940, treats the sale of substandard drugs as a serious offence. The law calls for a jail sentence of a minimum of one year and fines for the manufacturer; for the entire batch of the drug to be recalled from the market; and for the manufacturer to conduct a root-cause analysis into how the faulty drug got made.
Drugs made in India are sparking safety concerns
- India is the world's largest exporter of generic drugs, meeting much of the medical needs of developing countries.
- But in recent months, many Indian firms have come under scrutiny for the quality of their drugs, with experts raising concerns
- about the manufacturing practices used to make these medicines.
- At least five of the India-manufactured drugs have come under WHO scrutiny in close to a year.
- India-made cough syrups were allegedly linked to the deaths of 66 and 18 children in The Gambia and Uzbekistan, respectively, last year. Another alleged episode of cough-syrup related contamination was recently reported from Cameroon.
- In the US, children suffered severe eye infection allegedly after using India-made eye drops.
How bad is India’s substandard and counterfeit drug problem?
- According to a 2017 WHO report, about 5 per cent of medicines sold in low and middle-income countries, including India, are substandard and falsified. A report by United States Trade Representative (USTR) said that 20 per cent of all pharmaceutical products sold in the Indian market are counterfeit.
- In 2018, the national drugs regulatory body identified about 5 per cent of all generic drugs in the Indian market to be substandard.
- In February 2020, several batches of generic medicines being sold at Jan Aushadi Kendras, some 632 in Karnataka, and more pan-India were recalled after failing standard quality tests of state drug controller.
- In 2019-2020, India recalled four drugs and 27 batches.
- According to a report by Authentication Solutions Providers’ Association (ASPA), a non-profit organisation dealing in the area of anti-counterfeit awareness, the incidents of sub-standard and falsified (SF) drugs and other medical products related to COVID-19 increased by almost 47 per cent just within a year – from 2020 to 2021.
- The biggest concern, of course, remains adulterated drugs. In 2020, about 13 children, all under five years of age, died after drinking adulterated cough syrup. Earlier that year, around a dozen children from a village in Jammu, succumbed after drinking another diethylene-glycol-laden tonic, called Coldbest, reports Mint.
- In 1986, 14 patients died after treatment with diethylene-glycol-tainted glycerine in Mumbai’s Jamshetjee Jejeebhoy Hospital.
- Some of the main reasons for the large counterfeit medicines market in India include:
- limited access to medical care, especially in rural areas
- fragmented supply chain
- lack of consumer awareness
- prevalent practice of self-medication
- high cost of genuine medicines
- weak enforcement of legislation and corruption
- prevalence of internet pharmacies
- technology advancements in counterfeiting
- Most counterfeit medicines are taken unknowingly, as detecting counterfeit medicines is difficult, even for health care professionals.

How is our reputation in the world?
- India is one of the largest exporters of generic medicines. Forty per cent of over-the-counter and prescription generics sold in the US come from India. In the UK, a quarter of all generics come from India, and generics account for 80 per cent of National Health Service prescriptions.
- A special “301 Report” by United States Trade Representative published in 2019 said that India was a leading source of counterfeit medicine distributed locally. The report claimed that India exports counterfeit drugs to Africa, Canada, the Caribbean, the European Union, South America and the United States.
- However, India has rejected the allegations, saying it was an attack on low-cost generic drugs which are crucial to making healthcare affordable
Where does India go wrong?
- Under the National Good Laboratory Practice programme, India has only 47 drug testing facilities and six central labs, testing just 8,000 samples per year. Moreover, India has only 20-30 test laboratories that can affirm whether a drug is counterfeited, authentic or of relatively poor make.
- Mahesh Zagade, who headed the Maharashtra Food and Drugs Administration between 2011 and 2014, told that the Drug Consultative Committee’s guidelines “are designed to protect the industry, not to safeguard the patient”.
Challenges posed by counterfeit medicines
- The large counterfeit trade in India has created a number of complex challenges for the health care and life science industries.
Lost business opportunities:
- The presence of counterfeits can result in the loss of market share and business opportunities for manufacturers of genuine pharmaceutical products.
- It is estimated that counterfeit medicines contribute to a loss of $46 billion annually to pharmaceutical companies worldwide.
Undermining the adoption of generics:
- An estimated 90 per cent of the value of India’s drug market is dominated by branded generics.8 In order to reduce health care costs, many governments promote the use of less expensive unbranded generic medicines, but the availability of counterfeits is an obstacle to uptake.
Increasing the economic and social burden:
- The use of counterfeit medicines results in an increase in cost to the health care system due to the need for further interventions for unwanted side effects and/or advanced disease progression.
- This is a particular issue for Indians, where out of pocket drug spending is already high at almost 70 per cent, and affordability levels are low
Resourcing:
- To tackle the issue of counterfeits, the Indian government has employed various anti-counterfeiting strategies, but with limited impact, largely due to India’s Central Drugs Standard Control Organization, the country’s drug regulator, having only 323 employees in 2014, about two percent the size of the FDA.
- This under-resourcing is likely to undermine the success of any future strategies.
Strategies to overcome the issue of counterfeit medicines
There are a number of strategies that can be implemented to tackle the issue of counterfeit medicines.
Raising Public Awareness:
- Containing the spread of counterfeit medicines is a big challenge, but educating patients and improving awareness is a good place to start. Health care professionals, medical practitioners, and pharmaceutical companies all have a role to play in notifying patients and educating them to detect the presence of counterfeits.
- This is a sizeable challenge given that over a quarter of the population is illiterate, and almost 70 percent of the population live in rural areas where self-medication is prevalent.
- However, approximately 78 per cent of India’s 650 million mobile phone users have access to the internet, and online education may be a far more effective way to tackle the issue quickly and efficiently.
Implementing innovative anti-counterfeiting measures:
- Inadequate anti-counterfeiting measures by pharmaceutical companies make their products vulnerable to counterfeiting. The presence of counterfeit medicine brands in the market lead to drug recalls and subsequent damage to brand image and company reputation.
- Most companies are following worldwide mandatory methods like mass serialization in which machine-readable codes containing a serial number are added to each pack of medicines, enabling the product to be tracked and traced.
- Pharmaceutical companies can fight the rise of counterfeiting by implementing new generation anti-counterfeiting technologies, such as the use of forensic markers (chemical, biological and DNA taggants), cloud-based supply chain data repositories, and blockchain technology in supply chains.
- Companies can also use external providers of anti-counterfeiting technologies to avoid the need to create their own systems.
Developing stricter laws:
- In India, The Drugs and Cosmetics Act (1940) regulates the import, manufacturing and distribution of drugs, empowering government agencies to inspect, seize and confiscate products found to be ‘adulterated spurious misbranded’.
- The act was amended in 2008, which increased the punishment to offenders, but there are still certain challenges, including inconsistent application of laws across states, weak drug quality investigation systems, and weak prosecution of counterfeit medicine manufacturers. According to the WHO, 50 per cent of drugs sold online are fraudulent.13 New protocols which the EU and US aim to implement by 2019 are intended to tackle this
- . The EU’s Falsified Medicines Directive will require companies to adopt mass serialisation, whereas the US Drug and Security Act will require authentication at every supply chain juncture. The EU is also fighting illegal online pharmacies that supply counterfeit medicines by requiring them to display a logo identifying themselves. This logo links to the website of the national competent authority listing all legally operating online pharmacies. More effective adherence to existing laws and adopting stricter laws has the potential to improve the fight against counterfeiting.
Way Ahead
- Experts say that there need to be more stringent guidelines to stop such malpractices.
- WHO requested increased surveillance and diligence within the supply chains of countries and regions likely to be affected by these products.
- It also advised increased surveillance of informal and unregulated markets.
- National regulatory authorities and health authorities have been advised to immediately notify WHO if these substandard products are discovered in their respective country.
- Manufacturers of liquid dosage forms, especially syrups that contain excipients including propylene glycol, polyethylene glycol, sorbitol, and/or glycerin/glycerol, are urged to test for the presence of contaminants such as ethylene glycol and diethylene glycol before use in medicines.
- Healthcare professionals have been advised to report any suspicious cases of adverse events linked to the use of contaminated medicines to the National Regulatory Authorities or National Pharmacovigilance Centre.
.jpg)
Conclusion
- Global pharma companies are looking to India as a future growth prospect due to its fast growing economy, ageing population, growing middle-class, increasing presence of chronic diseases, inexpensive labour, and improving health care provision.
- However, the counterfeiting crisis is putting India at risk of losing foreign investments.
- Improvements to the regulatory landscape, and stricter punishments for manufacturers of counterfeits could significantly improve investor sentiment.
- Without strong rhetoric and action, India risks losing valuable investment from the pharmaceutical industry, and consequently, undermine the health of its population.
|
PRACTICE QUESTION
Q. How bad is India’s substandard and counterfeit drug problem? Outline strategies to overcome the issue of counterfeit medicines.
|
https://www.reuters.com/business/healthcare-pharmaceuticals/who-flags-contaminated-india-made-syrup-iraq-2023-08-07/













