Description
Disclaimer: Copyright infringement not intended.
Context
- An EV battery explosion has claimed one life and left two others injured in Hyderabad. Police have registered an FIR against the e-scooter manufacturer Pure EV.
- E-scooters from Okinawa Scooters, Ola Electric, Pure EV, and Jitendra Electric Vehicles have gone up in flames in recent weeks, putting the electric vehicle industry under pressure.
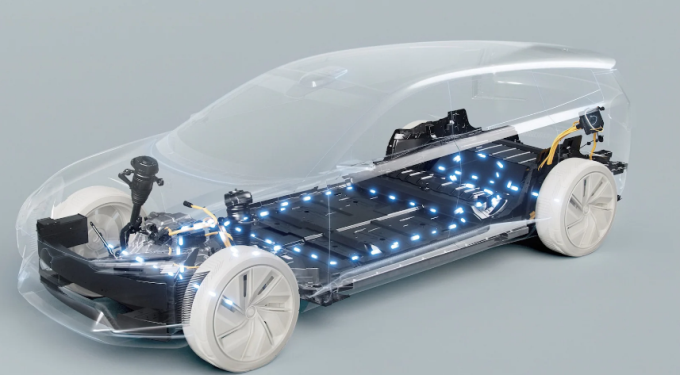
Electric vehicle battery
- An electric vehicle battery (EVB, also known as a traction battery) is a rechargeable battery used to power the electric motors of a battery electric vehicle (BEV) or hybrid electric vehicle (HEV).
- Typically lithium-ion batteries, are specifically designed for high electric charge (or energy) capacity.
Battery
- A battery is a device for storing chemical energy and converting that chemical energy into electricity.
- A battery is made up of one or more electrochemical cells, each of which consists of two half-cells or electrodes.
- One half-cell, called the negative electrode, has an overabundance of the tiny, negatively charged subatomic particles called electrons. The other, called the positive electrode, has a deficit of electrons.
- When the two halves are connected by a wire or an electrical cable, electrons will flow from the negative electrode to the positive electrode. We call this flow of electrons electricity.
- The energy of these moving electrons can be harnessed to do work -- running a motor, for instance.
- The electrons are generated by chemical reactions, and there are many different chemical reactions that are used in commercially available batteries. For example, the familiar alkaline batteries commonly used in flashlights and television remote controls generate electricity through a chemical reaction involving zinc and manganese oxide. Most alkaline batteries are considered to be a disposable battery. Once they go dead, they're useless and should be recycled.
- Automobile batteries, on the other hand, need to be rechargeable, so they don't require constant replacement. In a rechargeable battery, electrical energy is used to reverse the negative and positive halves of the electrochemical cells, restarting the electron flow.
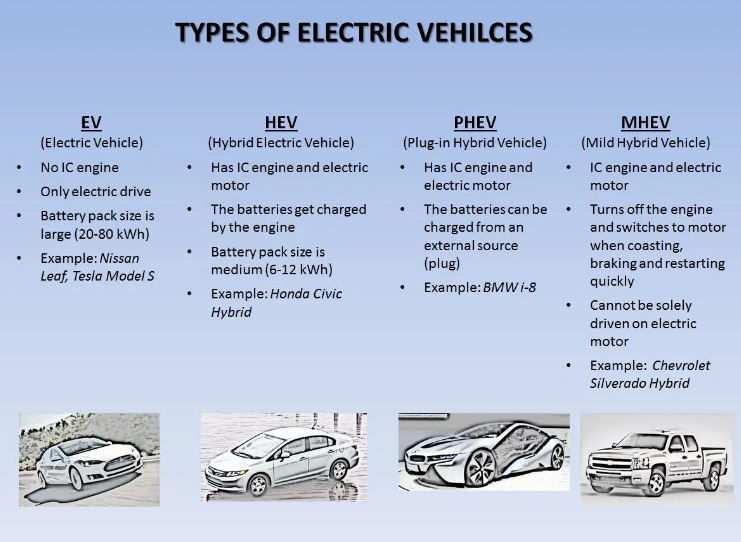
Types of Batteries used in automobiles
- Automobile manufacturers have identified three types of rechargeable battery as suitable for electric car use.
- Those types are lead-acid batteries, nickel metal hydride (NiMH) batteries, and lithium-ion (Li-ion) batteries.
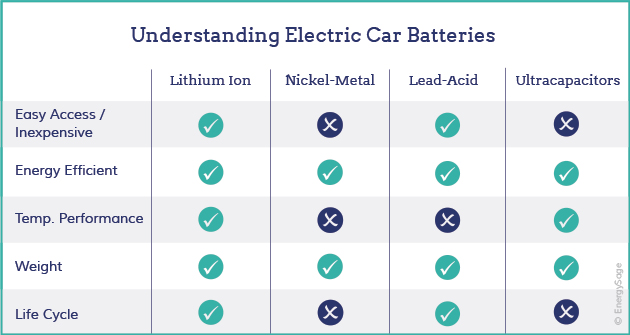
Lead-acid batteries
- Lead-acid batteries were invented in 1859 and are the oldest form of rechargeable battery still in use.
- Lead-acid batteries are a kind of wet cell battery and usually contain a mild solution of sulfuric acid in an open container.
- The name comes from the combination of lead electrodes and acid used to generate electricity in these batteries.
- Lead-acid batteries are only currently being used in electric vehicles to supplement other battery loads.
- These batteries are high-powered, inexpensive, safe, and reliable, but their short calendar life and poor cold-temperature performance make them difficult to use in electric vehicles.
- There are high-power lead-acid batteries in development, but the batteries now are only used in commercial vehicles as secondary storage.
Nickel metal hydride batteries
- Nickel metal hydride batteries came into commercial use in the late 1980s. They have a high energy density -- that is, a great deal of energy can be packed into a relatively small battery -- and don't contain any toxic metals, so they're easy to recycle.
- Nickel-metal hydride batteries are more widely used in hybrid-electric vehicles, but are also used successfully in some all-electric vehicles.
- Nickel-metal hydride batteries have a longer life-cycle than lithium-ion or lead-acid batteries.
- The biggest issues with nickel-metal hydride batteries are their high cost, high self-discharge rate, and the fact that they generate significant heat at high temperatures.
- These issues make these batteries less effective for rechargeable electric vehicles, which is why they are primarily used in hybrid electric vehicles.
Lithium-ion batteries
- Lithium-ion batteries, which came into commercial use in the early 1990s, have a very high energy density and are less likely than most batteries to lose their charge when not being used -- a property called self discharge.
- Because of their light weight and low maintenance requirements, lithium-ion batteries are widely used in electronic devices such as laptop computers.
- Some experts believe that lithium-ion batteries are about as close as science has yet come to developing a perfect rechargeable battery, and this type of battery is the best candidate for powering the electric cars of the near future. These batteries are also used in most portable electronics, including cell phones and computers.
- Lithium-ion batteries have a high power-to-weight ratio, high energy efficiency and good high-temperature performance.
- In practice, this means that the batteries hold a lot of energy for their weight, which is vital for electric cars – less weight means the car can travel further on a single charge.
- Lithium-ion batteries also have a low “self-discharge” rate, which means that they are better than other batteries at maintaining the ability to hold a full charge over time.
- Additionally, most lithium-ion battery parts are recyclable making these batteries a good choice for the environmentally conscious.
- The major advantage of lead-acid batteries is that, they are cheap to produce. However, they do produce dangerous gases while being used and if the battery is overcharged there's a risk of explosion.
|
A variation on lithium-ion batteries, called lithium-ion polymer batteries, may also prove valuable to the future of EVs. These batteries may eventually cost less to build than lithium-ion batteries; however, at the present time, lithium-ion polymer batteries are prohibitively expensive.
|
Ultracapacitors
- Ultracapacitors are not batteries in the traditional sense. Instead, they store polarized liquid between an electrode and an electrolyte.
- As the liquid’s surface area increases, the capacity for energy storage also increases. Ultracapacitors, like lead-acid batteries, are primarily useful as secondary storage devices in electric vehicles because ultracapacitors help electrochemical batteries level their load.
- In addition, ultracapacitors can provide electric vehicles with extra power during acceleration and regenerative braking.
https://www.thehindu.com/business/Industry/gadkari-warns-of-heavy-penalties-and-vehicle-recalls-post-ev-explosion/article65342341.ece