Description

Disclaimer: Copyright infringement not intended.
Context
- Physicists in Japan discovered a previously unknown isotope of uranium, with atomic number 92 and mass number 241, i.e. uranium-241.
Background
- Over the past several decades, physicists have found that determining the properties of neutron-rich isotopes is difficult due to problems caused in creating them.
- For that reason, ongoing research has been looking for new ways to synthesize them under lab conditions.
Why does a new isotope matter?
- The arrangement of protons and neutrons in an atomic nucleus follows some rules. These rules are based on the nuclei’s properties and structure.
- “In general, an atom’s mass is slightly lower than the sum of the masses of protons, neutrons, and electrons.
- So systematically measuring the mass of uranium and its neighborhood elements yields essential nuclear information to understand the synthesis of such heavy elements in explosive astronomical events.
.jpeg)
The new Research Techniques
Multinucleon Transfer
- In this new effort, the research team tried a new approach—they fired a sample of unranium-238 nuclei at a sample of platinum-198 nuclei using an isotope separation system.
- Such interactions are known to result in multinucleon transfer, in which isotopes swap neutrons and protons.
- The collision resulted in the creation of a large number of fragments, which the researchers studied to determine their makeup.
Time-of-Flight Mass Spectrometry
- They found evidence of 19 heavy isotopes holding from 143 to 150 neutrons.
- Each was measured using time-of-flight mass spectrometry, a technique that involves determining the mass of a traveling ion by tracking the time it takes to travel a given distance when its initial acceleration is known.
Findings
- The research team noted that most of the isotopes they measured had never been measured before.
- They also noted that one of them, uranium-241, had never been observed before and that it marks the first time since 1979 that a neutron-rich uranium isotope has been discovered.
- The researchers also calculated that uranium-241 likely has a half-life of just 40 minutes.

Significance
- The technique used by the team represents a pathway to better understanding the shapes of large nuclei associated with the heavy elements, which could yield changes to models used to build nuclear power plants and weapons and to theories describing the behavior of exploding stars.
Trivia
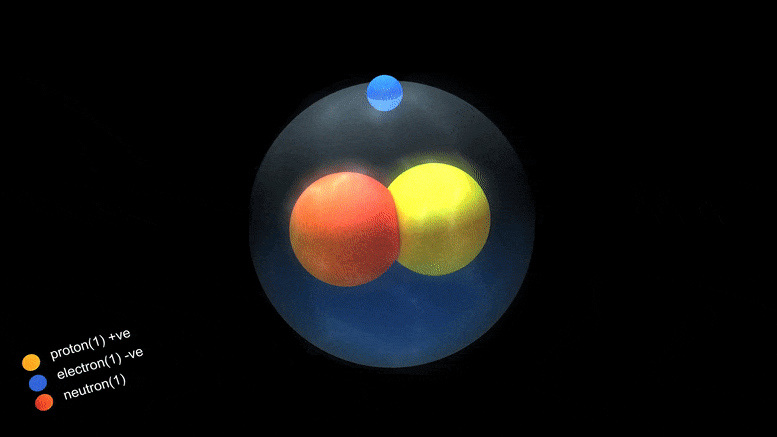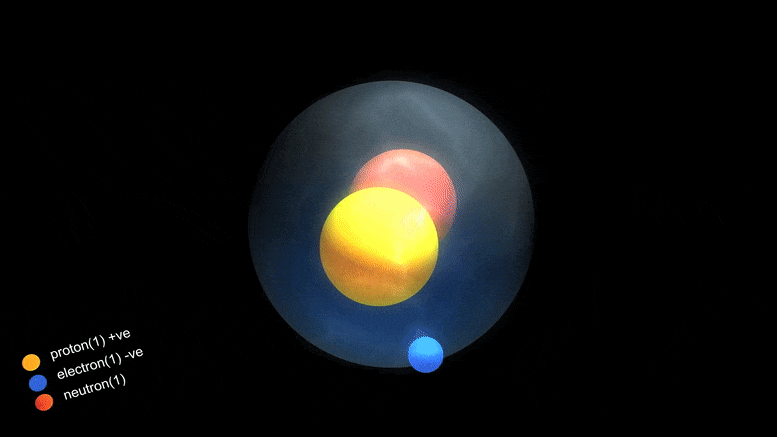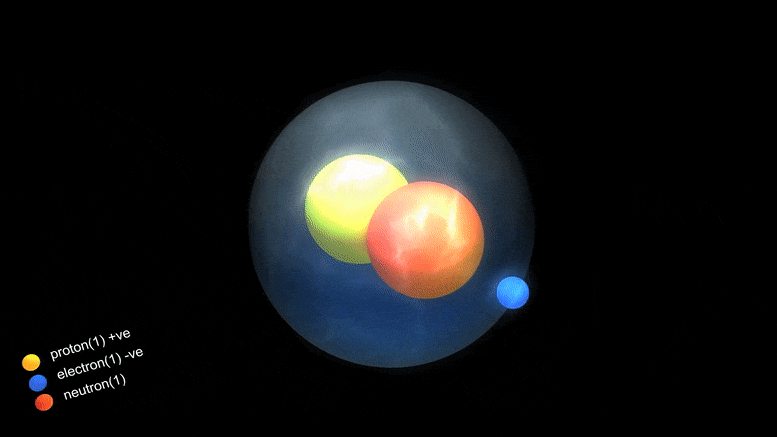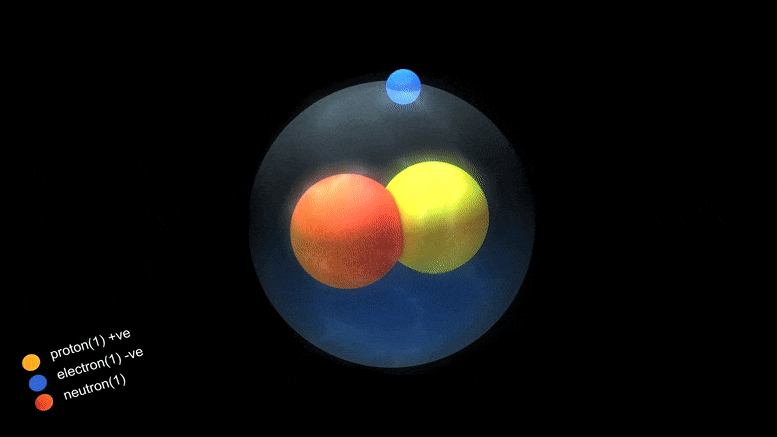
Isotopes
- Isotopes are members of a family of an element that all have the same number of protons but different numbers of neutrons.
- The number of protons in a nucleus determines the element’s atomic number on the Periodic Table. For example, carbon has six protons and is atomic number 6. Carbon occurs naturally in three isotopes: carbon 12, which has 6 neutrons (plus 6 protons equal 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. Every element has its own number of isotopes.
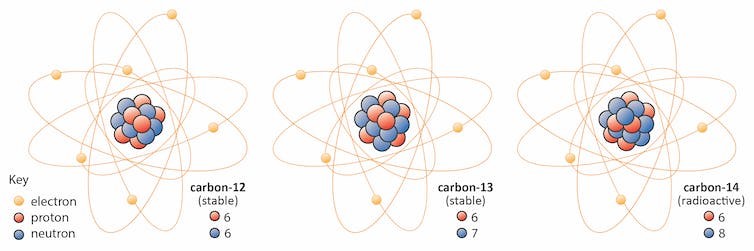
- The addition of even one neutron can dramatically change an isotope’s properties. Carbon-12 is stable, meaning it never undergoes radioactive decay. Carbon-14 is unstable and undergoes radioactive decay with a half-life of about 5,730 years (meaning that half of the material will be gone after 5,730 years). This decay means the amount of carbon-14 in an object serves as a clock, showing the object’s age in a process called “carbon dating.”
- Isotopes have unique properties, and these properties make them useful in diagnostics and treatment applications. They are important in nuclear medicine, oil and gas exploration, basic research, and national security.
Origin of Isotopes
- Some of the lighter isotopes were formed very early in the history of the universe, during the Big Bang. Others result from processes that happen within stars or as a result of chance collisions between highly energetic nuclei - known as cosmic rays- within our atmosphere.
- Most naturally existing isotopes are the final (stable or long-lived) product resulting from a long series of nuclear reactions and decays.
- In most of these cases, light nuclei have had to smash together with enough energy to allow the strong force- a glue-like bond that forms when protons and neutrons get close enough to touch - to overcome the electromagnetic force – which pushes protons apart. If the strong force wins out, the colliding nuclei bind together, or fuse, to form a heavier nucleus.
- Our sun is a good example of this. One of its main sources of power is a series of fusion reactions and beta decay processes that transform hydrogen into helium.
Isotope Facts
- All elements have isotopes.
- There are two main types of isotopes: stable and unstable (radioactive).
- There are 254 known stable isotopes.
- All artificial (lab-made) isotopes are unstable and therefore radioactive; scientists call them radioisotopes.
- Some elements can only exist in an unstable form (for example, uranium).
- Elements can have both stable and radioactive isotopes. Strontium, for example, has four stable isotopes: Sr-84, Sr-86, Sr-87, and Sr-88; and one radioactive isotope, Sr-82. Over time, Sr-82 decays to rubidium-82 with a half-life of 25 days, making it suitable for use in generators to provide rubidium-82, the most convenient positron emission tomography agent for heart imaging. Twenty-six elements only have one stable element, while tin has the most stable isotopes with ten.
- Hydrogen is the only element whose isotopes have unique names: deuterium for hydrogen with one neutron and tritium for hydrogen with two neutrons.
‘Magic Numbers’
- There is particular interest in ‘magic number’ nuclei: containing a number of protons orneutrons such that the resulting nucleus is highly stable. The heaviest known ‘magic’ nucleus is lead (82 protons).
- Physicists have been trying to find the next such element.
|
PRACTICE QUESTION
Q. Which of the following statements are correct in reference to Isotopes?
a) Multinucleon transfer is a phenomenon, in which isotopes swap neutrons and protons.
b) All artificial isotopes are unstable and therefore radioactive; scientists call them radioisotopes.
c) Carbon-12 is a stable isotope, meaning it never undergoes radioactive decay.
d) Elements can have both stable and radioactive isotopes.
1. a and b
2. b and d
3. a, c and d.
4. All of the above.
Correct Answer: Option 4
|

https://www.thehindu.com/sci-tech/science/in-pursuit-of-a-magic-number-physicists-discover-new-uranium-isotope/article66699249.ece


















