Description
Disclaimer: Copyright infringement not intended.
Context
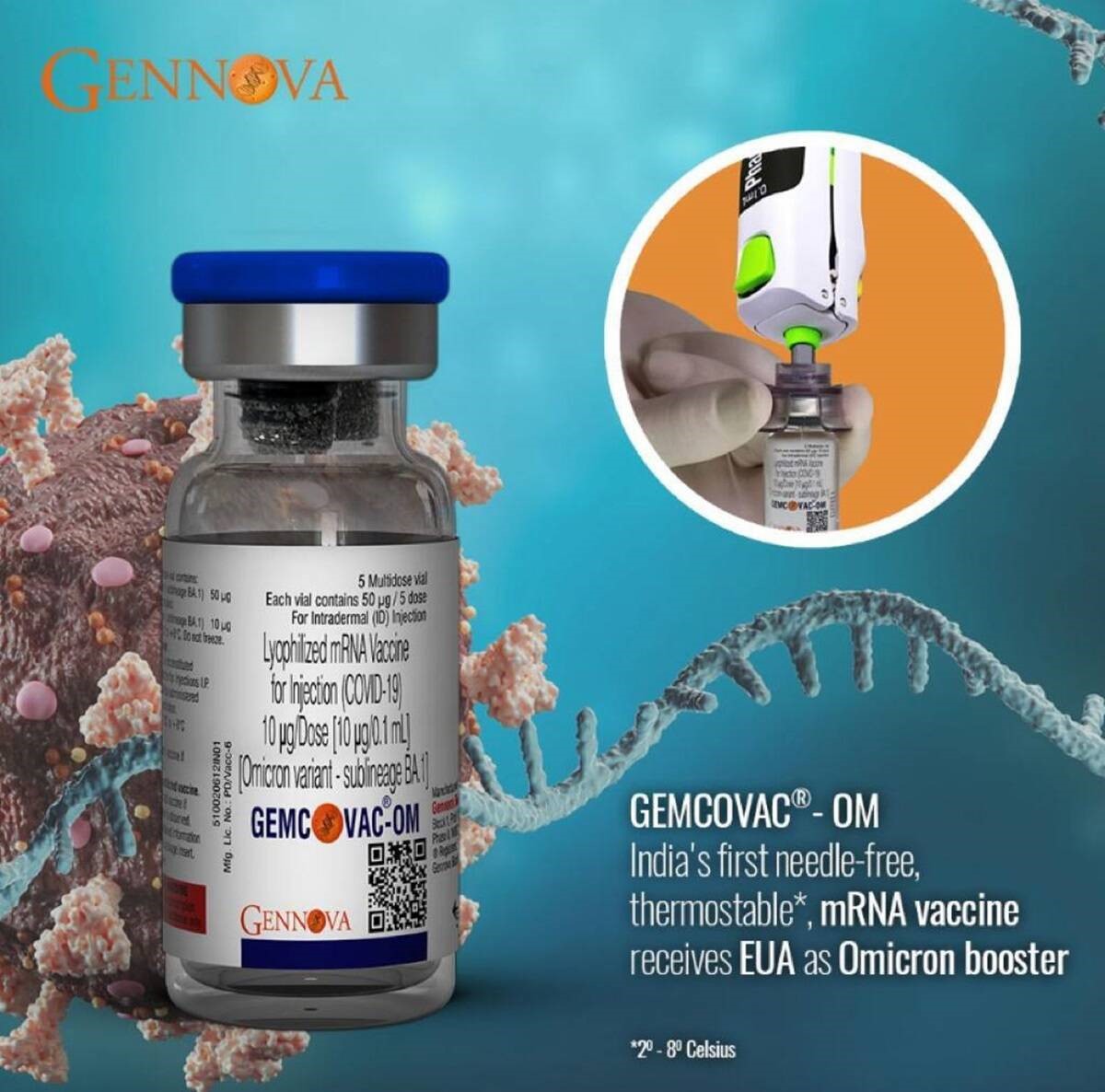
- Pune based Gennova Biopharmaceuticals announced that its mRNA COVID-19 booster vaccine – GEMCOVAC-OM - against the Omicron variant of SARS-CoV-2 received emergency use authorization (EUA) from the office of the Drugs Controller General of India (DCGI).
Background: How mRNA vaccine works?
- To trigger an immune response, many vaccines put a weakened or inactivated version of the virus into our bodies, just like Covaxin and Covishield.
- mRNA vaccines use mRNA created in a laboratory to teach our cells how to make a protein — or even just a piece of a protein — that triggers an immune response inside our bodies.
- Using this mRNA blueprint, cells produce the viral protein. As part of a normal immune response, the immune system recognises that the protein is foreign and produces specialised proteins called antibodies.
- Antibodies help protect the body against infection by recognising individual viruses and marking the pathogens for destruction.
- Once produced, antibodies remain in the body, even after the body has rid itself of the pathogen, so that the immune system can quickly respond if exposed again.
ALL ABOUT mRNA VACCINES: https://www.iasgyan.in/daily-current-affairs/explained-what-is-mrna-vaccine
https://iasgyan.in/daily-current-affairs/mrna-vaccine-29
GEMCOVAC-OM
- GEMCOVAC is an mRNA-based vaccine using a spike protein, a small piece of a protein found on the outer membrane of the Coronavirus.
- GEMCOVAC-OM is the first booster COVID-19 vaccine developed in India against the highly transmissible Omicron variant.
- GEMCOVAC-OM has demonstrated robust immune responses in the phase 3 clinical trial conducted at 20 centers across 13 cities in India In Phase-II/ III trials, approximately 3,000 individuals received GEMCOVAC-OM and the vaccine was safe and well tolerated, according to a company press release.
Precursor of GEMCOVAC-OM: GEMCOVAC-19
- GEMCOVAC-19, the precursor to GEMCOVAC-OM, was developed by Gennova Biopharmaceuticals with support from the Mission Covid Suraksha programme, implemented by Biotechnology Industry Research Assistance Council (BIRAC).
Need for developing GEMCOVAC-19
- The currently approved vaccines used as precautionary/ booster doses are designed against the ancestral strain of SARS-CoV-2. Although these will increase the antibody titers, their ability to neutralize the circulating Omicron variant of SARS-CoV-2 is limited.
- Developing antibodies and memory immune responses specific to the Omicron variant would reduce the probability of infection and hospitalization and prevent future waves of the pandemic. The made in India GEMCOVAC-OM specifically addresses this gap.
Features of GEMCOVAC-OM
- GEMCOVAC-OM is a lyophilized (freeze dried) vaccine, stable at 2- 8 °C.
- It is delivered intradermally using a device called Tropis, developed by PharmaJet, USA. This is a needle-free device that obviates the disadvantages of using a needle, such as a needle phobia, sharps disposal, and needle-stick injuries, to name a few.
Benefits of GEMCOVAC-OM over other Vaccines
- The first two vaccines that were made available for use in the United States — Pfizer-BioNTech and Moderna vaccines — were based on mRNA technology.
- However, the Indian mRNA vaccines have an edge over its international competitors as it does not require sub-zero temperatures for its storage.
- GEMCOVAC-19 is not stored in liquid form. This mRNA vaccine developed is available in the lyophilised (freeze-dried) or powdered form. The vaccine can be reconstituted using a diluent before administration.
- Like the prototype vaccine, GEMCOVAC-OM too is a thermostable vaccine that does not require ultra-cold chain infrastructure used for other approved mRNA-based vaccines, making it easy for deployment pan-India.
- It is delivered intradermally using a needle-free injection device system. When administered intradermally in participants as a booster, it generated significantly higher immune responses. The clinical outcome demonstrated the need for variant-specific vaccines for desired immune response.
Significance of mRNA Vaccines
- mRNA vaccines help in generating a strong immunity. It not only generates antibodies to fight the virus, but also produces immune cells that help in attacking the virus doubly,
- The efficacy of mRNA is also higher than other vaccines. It can be more effective against mutant variants of the Coronavirus.
- mRNA vaccine technology can be tweaked in case of emerging variants.
- Multiple mRNAs can be encoded in a single vaccine, which might help in targeting antigens produced from different variants.
India and mRNA Vaccines
- India has now developed not one but two mRNA vaccines against Covid-19 using this rapid disease-agnostic platform technology.
- Infrastructure to deploy vaccines in India at 2‑8°C exist today and this innovation is tailored for the existing established supply-chain Infrastructure.
READ ABOUT BIRAC: https://www.iasgyan.in/daily-current-affairs/bionest-bioincubation-centre
|
PRACTICE QUESTION
Q. Consider the following statements with respect to GEMCOVAC-OM:
1.GEMCOVAC-OM is the first booster COVID-19 vaccine developed in India against the highly transmissible Omicron variant.
2.GEMCOVAC is a DNA-based vaccine using a spike protein, a small piece of a protein found on the outer membrane of the Coronavirus.
3.This vaccine needs ultra-low temperature conditions for transport and storage.
4.This vaccine is available in the lyophilised (freeze-dried) or powdered form.
Which of the above statements is/are correct?
(a) 1 and 4 only
(b) 2 and 4 only
(c) 1, 3, and 4 only
(d) All of the above.
Correct Answer: (a) 1 and 4 only
|
https://indianexpress.com/article/health-wellness/indias-first-mrna-based-omicron-specific-booster-vaccine-approved-8675607/
















