CHAPERONE

Source: PIB
Disclaimer: Copyright infringement not intended.
Context
- A new way to study protein folding & associated chaperons that saves proteins from non-native interaction, could help understand what exactly triggers the folding.
- The understanding could help track progression of diseases like cancer, Parkinsons and Alzheimer’s.
Details
Chaperones
- Chaperones are a diverse group of proteins that play crucial roles in maintaining cellular homeostasis by assisting the folding, assembly, translocation, and degradation of other proteins within the cell.
- They are essential for the correct folding of newly synthesized proteins, the refolding of misfolded or aggregated proteins, and the prevention of protein aggregation under stress conditions.
Classification of Chaperones
Chaperones can be classified into several major families based on their structure, function, and mechanism of action:
- Heat Shock Proteins (HSPs)
- Hsp70 Family: These chaperones assist in protein folding and protect cells from stress-induced damage. They bind to nascent polypeptide chains and prevent premature folding.
- Hsp90 Family: These chaperones are involved in the final stages of protein folding and in the stabilization of proteins required for cell signaling.
- Hsp60 Family (Chaperonins): These chaperones form large, barrel-shaped complexes that provide an isolated environment for proteins to fold correctly.
- Small Heat Shock Proteins (sHSPs)
- These chaperones act as molecular buffers by binding to unfolded proteins and preventing their aggregation. They do not actively refold proteins but keep them in a soluble state until they can be refolded by other chaperones.
- Hsp100/Clp Family: These chaperones are involved in the disaggregation and refolding of aggregated proteins. They often work in conjunction with other chaperones like Hsp70.
- Co-chaperones: Co-chaperones regulate the activity of major chaperones. For example, Hsp40 (DnaJ) proteins act as co-chaperones for Hsp70, enhancing its ability to bind and release substrate proteins.
Functions of Chaperones
- Protein Folding: Chaperones assist newly synthesized polypeptides in attaining their correct three-dimensional structure. This is crucial for the functionality of the proteins.
- Prevention of Aggregation: Chaperones bind to partially folded or misfolded proteins, preventing their aggregation, which can be toxic to cells.
- Protein Refolding: Under stress conditions, such as heat shock, proteins may unfold or misfold. Chaperones help refold these damaged proteins back to their native state.
- Protein Degradation: Some chaperones are involved in targeting irreversibly misfolded proteins for degradation via the ubiquitin-proteasome system or autophagy.
- Translocation: Chaperones assist in the translocation of proteins across cellular membranes, such as from the cytosol into the mitochondria or endoplasmic reticulum.
Key Single Molecule Techniques
|
Technique |
Principle |
Applications |
Advantages |
Limitations |
|
Atomic Force Microscopy (AFM) |
Measures forces between a sharp probe and sample surface |
Imaging surfaces, measuring mechanical properties |
High resolution, versatile |
Limited to surface studies, slow scanning speed |
|
Optical Tweezers |
Uses laser beams to trap and manipulate small particles |
Studying molecular motors, force measurements |
High precision in force measurements |
Requires high laser power, potential photodamage |
|
Single-Molecule Fluorescence (SMF) |
Detects fluorescence from individual molecules |
Tracking molecular dynamics, interactions |
High sensitivity, real-time observation |
Photobleaching, background fluorescence |
|
Magnetic Tweezers |
Uses magnetic fields to apply force to magnetic particles |
Studying DNA-protein interactions, molecular mechanics |
Can apply force over a wide range, low photodamage |
Limited to magnetic materials, less precise than optical tweezers |
|
Patch-Clamp Technique |
Measures ion currents through individual ion channels |
Studying ion channel function, electrophysiology |
High temporal resolution, direct measurement |
Technically challenging, invasive |
|
Single-Molecule FRET (smFRET) |
Measures energy transfer between two fluorophores |
Studying conformational changes, interactions |
Provides distance information, high sensitivity |
Requires labeling with fluorophores, photobleaching |
|
Electron Microscopy (EM) |
Uses electron beams to image structures at high resolution |
Structural biology, material science |
Very high resolution, detailed structural information |
Requires vacuum, sample preparation can be complex |
|
Nanopore Sequencing |
Detects changes in ionic current as molecules pass through a nanopore |
DNA sequencing, analyzing biomolecules |
Direct reading of sequence, long read lengths |
Limited read accuracy, complex data analysis |
Proteins
- Proteins are large, complex molecules that play critical roles in the body.
- They are essential for the structure, function, and regulation of the body’s tissues and organs.
- Proteins are made up of hundreds or thousands of smaller units called amino acids, which are attached in long chains.
- There are 20 different types of amino acids that can be combined to make a protein
Structure of Proteins
Proteins have four levels of structure:
- Primary Structure: The primary structure of a protein is its unique sequence of amino acids. The sequence is determined by the gene encoding the protein.
- Secondary Structure: The secondary structure refers to local folded structures that form within a polypeptide due to interactions between atoms of the backbone. The most common types are alpha helices and beta-pleated sheets.
- Tertiary Structure: The tertiary structure is the overall three-dimensional structure of a polypeptide. It is determined by interactions among various side chains (R groups).
- Quaternary Structure: The quaternary structure is the structure formed by several protein molecules (polypeptide chains), usually called protein subunits
Types of Proteins
|
Protein |
Function |
Example |
|
Enzymes |
Catalyze biochemical reactions |
Amylase (digestion), DNA polymerase (replication) |
|
Structural |
Provide support and structure |
Collagen (connective tissue), Keratin (hair, nails) |
|
Transport |
Carry substances throughout the body |
Hemoglobin (oxygen transport), Albumin (nutrient transport) |
|
Motor |
Involved in movement |
Actin and Myosin (muscle contraction) |
|
Storage |
Store nutrients and other substances |
Ferritin (iron storage), Casein (milk protein) |
|
Signaling |
Coordinate bodily functions |
Insulin (regulates blood glucose levels), Hormones |
|
Receptor |
Receive and transmit signals |
G-protein coupled receptors (cell signaling) |
|
Defense |
Protect the body from pathogens |
Antibodies (immune response) |
Protein Synthesis
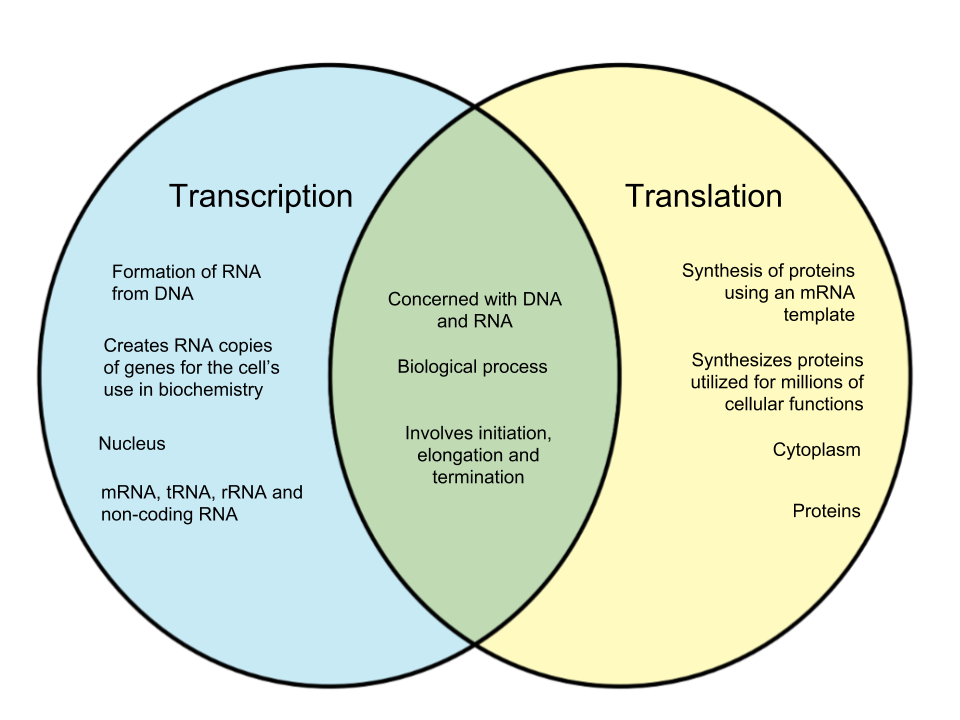
- Transcription: The process by which the information in a strand of DNA is copied into a new molecule of messenger RNA (mRNA). DNA safely and stably stores genetic material in the nuclei of cells as a reference or template.
- Translation: The process in which ribosomes in the cytoplasm or ER synthesize proteins after the process of transcription of DNA to RNA in the cell's nucleus. The mRNA is decoded to build a polypeptide, or chain of amino acids.
|
Protein Folding Protein folding is the physical process by which a protein chain acquires its native 3-dimensional structure, a conformation that is usually biologically functional. This process is crucial as the function of a protein is directly dependent on its structure. |
Functions of Proteins
- Catalysis: Enzymes are proteins that speed up chemical reactions in the body.
- Defense: Antibodies are proteins that help protect the body from diseases.
- Transport: Hemoglobin transports oxygen in the blood.
- Support: Structural proteins like collagen provide support in connective tissues.
- Motion: Motor proteins like actin and myosin are involved in muscle contractions.
- Regulation: Hormonal proteins like insulin regulate metabolism.
- Storage: Storage proteins like ferritin store iron in the liver.
Importance of Proteins in Nutrition
- Proteins are a vital part of a balanced diet.
- They provide the necessary amino acids that the body cannot synthesize on its own.
- Dietary proteins come from both animal and plant sources, and it is essential to consume a variety of protein sources to ensure adequate intake of all essential amino acids.
Protein-Related Disorders
- Enzyme Deficiency Disorders: Diseases caused by the lack of specific enzymes. Example: Phenylketonuria (PKU).
- Misfolded Protein Disorders: Diseases caused by the accumulation of misfolded proteins. Example: Alzheimer’s disease, Parkinson’s disease.
- Nutritional Deficiencies: Conditions caused by inadequate protein intake. Example: Kwashiorkor, Marasmus.
- Genetic Disorders: Diseases caused by mutations in genes encoding for proteins. Example: Cystic fibrosis.
Sources:
|
PRACTICE QUESTION Q: With reference to proteins, consider the following statements:
Which of the statements given above is/are correct? (a) 1 and 2 only (b) 2 and 3 only (c) 1 and 3 only (d) 1, 2, and 3 Answer: (a) |





1.png)
