Corbevax
Disclaimer: Copyright infringement not intended.
Context:
- India’s first indigenously developed Receptor Binding Domain (RBD) protein sub-unit vaccine for COVID-19, CORBEVAXTM, has received approval for Emergency Use Authorization from the Drug Controller General of India (DCGI)for 12-18 years age group.
- It is approved to be administered in humans including Children and adults 12 years and above.
About Corbevax:
- Corbevax has the distinction of being the first indigenously developed RBD protein sub-unit vaccine in India.
- Corbevax, as mentioned earlier is a protein subunit vaccine.
- The recombinant protein sub-unit vaccine developed from the Receptor Biding Domain (RBD) of the spike protein on the viral surface is adjuvanted with CpG 1018 and alum.
Vaccine Administration:
- The vaccine should be administered intramuscularly in two doses of 0.5 ml each with an interval of 28 days (Day 0 and 28) and has to be stored between 2 degrees Celsius to 8 degrees Celsius.
Development:
- Biological E. Limited (BE), a Hyderabad-based Pharmaceuticals & Biologics Company developed it.
- The Department of Biotechnology (DBT) and its Public Sector Undertaking (PSU), Biotechnology Industry Research Assistance Council (BIRAC), have played a key role in its development.
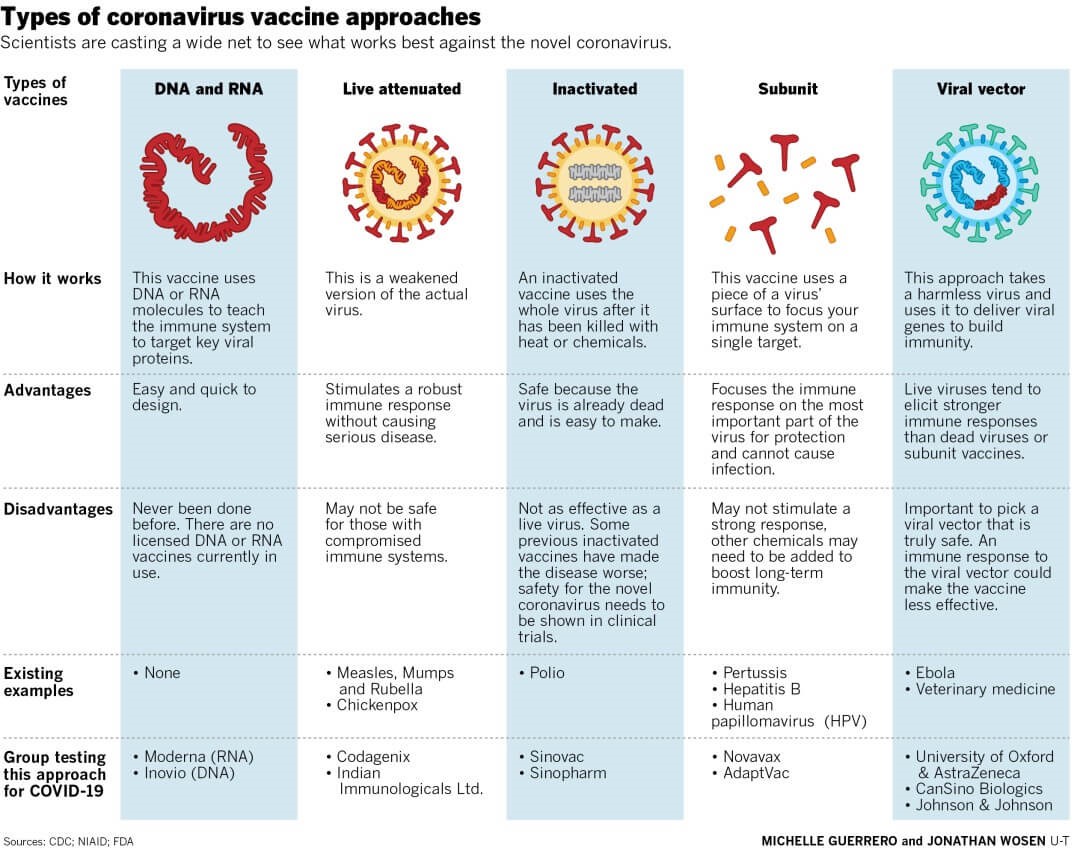
Types of Vaccine Platforms:
- There are two categories of Vaccines: Component Viral Vaccines and Whole Virus Vaccines.
- Component Viral Vaccines
- Protein Subunit: Contains isolated and purified viral proteins.
- Virus-like Particles (VLP): Contains viral proteins that mimic the structure of the virus, but no genetic material
- DNA-basedand RNA-based: Contains viral genetic material (such as mRNA) which provides the instructions for making viral proteins
- Non-Replicated Viral Vector: Contains viral genetic material packaged inside another harmless virus that cannotcopy itself
- Replicating Viral Vector: Contains viral genetic material packaged inside another harmless virus that cancopy itself.
Whole Virus Vaccines:
- Inactivated: Contains copies of the virus that have been killed (inactivated)
- Live-Attenuated: Contains copies of the virus that have been weakened (attenuated)



1.png)
