OMISURE
Disclaimer: Copyright infringement not intended.
Context
- The Indian Council of Medical Research (ICMR) has approved a testing kit for detecting the Omicron variant of SARS-CoV-2.
About
- The kit is manufactured by Tata Medical and Diagnostics and is named OmiSure.
- The kit will be used to confirm Omicron in patients with its S-Gene Target Failure (SGTF) strategy.
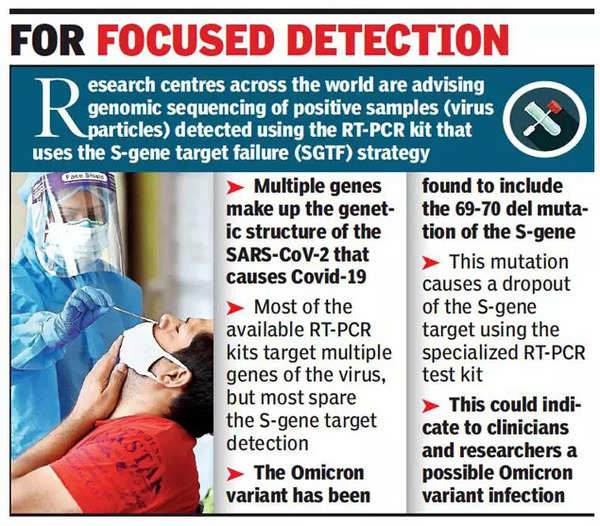
S-Gene Target Failure (SGTF)
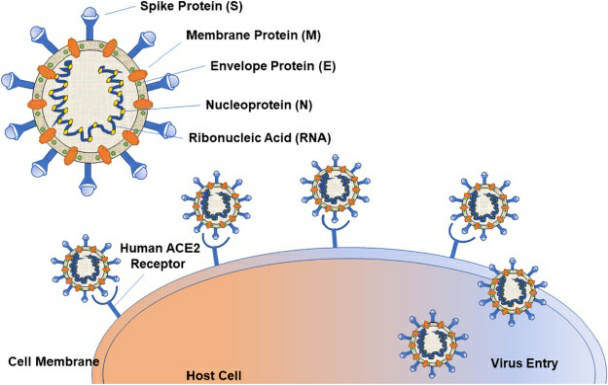
- Covid-19 Tests target "multiple genes" of the virus so that a broad range of variants are covered.
- For example, 'S' Gene, ORF, 'N' gene, Rdrp, 'E' gene etc are viral genes that are targeted to detect COVID-19 virus, and multiple genes make up the genetic structure of SARS-Co V-2.
- In case of Omicron variant, the 'S' gene is not getting detected in the test due to mutation in the gene, while other gene targets such as ORF gene and N gene are getting detected.
- The occurrence is called as 'S' Gene Target Failure (SGTF) positive cases.
- Such samples can be presumptively reported as Omicron positive and can be sent for fast-track genome sequencing for confirmation.
Significance
- Diagnostic services are critical for public health.
- During an outbreak, timely information is needed to guide and tailor public health response to stop/ curtail disease spread.
- The SGTF strategy will work as a kind of early detection at RT-PCR stage, and will help in screening COVID-19 positive samples of Omicron variant.
https://epaper.thehindu.com/Home/ShareArticle?OrgId=G2K9C4JDI.1&imageview=0



1.png)
